Volume 19, Number 8—August 2013
CME ACTIVITY - Research
Extended-Spectrum β-Lactamase–producing Enterobacteriaceae among Travelers from the Netherlands
Introduction

Medscape, LLC is pleased to provide online continuing medical education (CME) for this journal article, allowing clinicians the opportunity to earn CME credit.
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and Emerging Infectious Diseases. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit(s)TM. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 70% minimum passing score and complete the evaluation at www.medscape.org/journal/eid; (4) view/print certificate.
Release date: July 23, 2013; Expiration date: July 23, 2014
Learning Objectives
Upon completion of this activity, participants will be able to:
• Distinguish the rate of acquisition of multidrug-resistant Enterobacteriaceae (MDR-E) after foreign travel
• Analyze geographic areas associated with the highest risk for acquisition of MDR-E
• Evaluate other risk factors for the acquisition of MDR-E during travel
• Assess characteristics of extended-spectrum β-lactamase-producing Enterobacteriaceae in the current study.
CME Editor
Jean Michaels Jones, Technical Writer/Editor, Emerging Infectious Diseases. Disclosure: Jean Michaels Jones has disclosed no relevant financial relationships.
CME Author
Charles P. Vega, MD, Health Sciences Clinical Professor; Residency Director, Department of Family Medicine, University of California, Irvine. Disclosure: Charles P. Vega, MD, has disclosed no relevant financial relationships.
Authors
Disclosures: Sunita Paltansing, Jessica A. Vlot, Margriet E.M. Kraakman, Romy Mesman, Marguerite L. Bruijning, Alexandra T. Bernards, Leo G. Visser, and Karin Ellen Veldkamp have disclosed no relevant financial relationships.
Abstract
A prospective cohort study was performed among travelers from the Netherlands to investigate the acquisition of carbapenemase-producing Enterobacteriaceae (CP-E) and extended-spectrum β-lactamase–producing Enterobacteriaceae (ESBL-E) and associated risk factors. Questionnaires were administered and rectal swabs were collected and tested before and after return. Of 370 travelers, 32 (8.6%) were colonized with ESBL-E before travel; 113 (30.5%) acquired an ESBL-E during travel, and 26 were still colonized 6 months after return. No CP-E were found. Independent risk factors for ESBL-E acquisition were travel to South and East Asia. Multilocus sequence typing showed extensive genetic diversity among Escherichia coli. Predominant ESBLs were CTX-M enzymes. The acquisition rate, 30.5%, of ESBL-E in travelers from the Netherlands to all destinations studied was high. Active surveillance for ESBL-E and CP-E and contact isolation precautions may be recommended at admission to medical facilities for patients who traveled to Asia during the previous 6 months.
The effect of international travel on the spread of multidrug-resistant Enterobacteriaceae (MDR-E) became more evident during 2007–2010. Data obtained during that time from prospective studies of returning travelers from Australia, Canada, Sweden, and the United States (New York, New York) revealed high rates of extended-spectrum β-lactamase producing Enterobacteriaceae (ESBL-E) carriage, varying from 18% to 25% after foreign travel (1–4). Two of these studies also reported a pretravel ESBL-E carriage rate of 7.8%.
The identification of carbapenemase-producing Enterobacteriaceae (CP-E) produced another set of challenges. Carbapenemases, such as Klebsiella pneumoniae carbapenemases (KPC), New Delhi metallo-β-lactamase (NDM), OXA-48, VIM and IMP, are plasmid-encoded enzymes, which have emerged worldwide. The rate of acquisition of CP-E during foreign travel is unknown; no surveillance system to date tracks these rates, and such rates are included sporadically in case reports, such as the situation recently reviewed by Van der Bij and Pitout (5). In the Netherlands, CP-E were found for the first time in 2010 (6).
No data were available on the pre-and post-travel carriage rates among travelers from the Netherlands. Our objective was to investigate whether these travelers are at risk of MDR-E (ESBL-E and/or CP-E) by use of a prospective cohort study design. Because detailed microbiological data of the isolates and epidemiologic data are crucial for assessing the real public health impacts of these organisms, we also investigated the persistence of intestinal colonization and possible spread to household contacts 6 months after the travelers returned.
Study Design
A prospective cohort study was conducted at the travel clinic at the Leiden University Medical Center and at the Hollands Midden Municipal Health Services in Leiden, the Netherlands. During March–September 2011, all adults who made an appointment for travel advice and had the intention to travel to areas outside Europe, North America, and Australia were invited to participate in the study. Travelers <18 years of age and those who traveled >3 months were excluded. Only 1 person in a couple or travel group was included.
Participants were asked to complete an electronic questionnaire and to deliver a rectal swab sample immediately before and immediately after travel. Questionnaires were used to collect demographic data, previous medical history, and travel information. Travelers who acquired MDR-E after foreign travel were asked to fill out a third questionnaire and deliver a third rectal swab 6 months after return.
If travelers were positive for MDR-E 6 months after return, their household contacts were also requested to submit a rectal swab and questionnaire. Household contacts were defined as persons who shared the same household with a participant on a regular basis. MDR-E–positive participants were asked to deliver a fourth rectal swab at the same time. The study was approved by the Leiden University Medical Center medical ethics committee.
Bacterial Isolates
Rectal swab samples were collected with Stuart Agar Gel Medium Transport Swabs (Copan Diagnostics, Corona, CA). The swabs were inoculated in trypticase soy broth supplemented with cefotaxime 0.25 mg/L and vancomycin 8 mg/L (MP products, Groningen, the Netherlands) and incubated for 24 hours at 37°C. After overnight incubation, the trypticase soy broth samples were subcultured on chromogenic ESBL screening agar (ESBL-ID; bioMérieux, Marcy-l’Étoile, France) and sheep blood agar as a growth control. All gram-negative rods growing on the ESBL-ID were identified by using MaldiTof-MS with BioTyper software version 3.0 (Bruker Daltonics, Breman, Germany), and antimicrobial drug susceptibility testing was performed by using the VITEK2 system (BioMérieux). All isolates underwent ESBL confirmatory disk testing by disk diffusion for ceftazidime and cefotaxime or cefepime (in cefoxitin-resistant isolates), with and without clavulanic acid, as recommended by Clinical and Laboratory Standards Institute guidelines (www.clsi.org).
MICs for meropenem and ertapenem were determined by using Etests (AB Biodisk, Solna, Sweden) according to the manufacturer’s instructions. MICs were interpreted by using EUCAST criteria (www.eucast.org/clinical_breakpoints/).
Molecular Characterization of β-Lactamases
Molecular characterization of the β-lactamase genes in ESBL-E was performed by using Check-MDR CT103 version 1.1 (Check-Points B.V., Wageningen, the Netherlands) to test microarrays. The principals of the microarray system and interpretation software have been described (7). Concisely, the system combines ligation-mediated amplification with the detection of amplified products on a microarray to detect the various carbapenemase genes: OXA-48, NDM-1, IMP, VIM, and KPC; CTX-M groups: CTX-M group 1, 2, 9 or combined 8/25; and the most prevalent ESBL-associated single-nucleotide polymorphisms in TEM and SHV-variants. Furthermore, the 6 plasmid-mediated AmpC β-lactamases can be identified (www.lahey.org/studies).
Molecular Typing of Escherichia coli isolates
Multilocus sequence typing (MLST) was performed on all E. coli isolates by using 7 housekeeping genes (adk, fumC, gyrB, icd, mdh, purA, and recA) to determine the corresponding sequence type (ST) and to designate the sequence type complex (STC) by using the MLST Databases at the Environmental Research Institute, University College Cork website (http://mlst.ucc.ie/mlst/dbs/Ecoli).
Data analysis
A logistic regression model was used to determine risk factors for the acquisition of ESBL-E/CP-E after foreign travel for a total of 338 participants. Associations between acquiring an ESBL-E/CP-E after travel and different variables are calculated as odds ratios and p-values. Participants who were positive for ESBL-E/CP-E before travel were analyzed separately. Database processing and statistical analyses (univariate and multivariate analysis) were performed by using the SPSS software version 20.0 (SPSS Inc., Chicago, IL, USA). MLST analysis was performed by using BioNumerics software v.6.6 (Applied Maths, St-Martens-Lathem, Belgium).
Study Population and Travel Characteristics
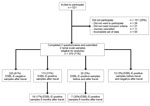
In total, 521 travelers were invited to participate in the study; 370 travelers completed 2 questionnaires and sent in 2 rectal swabs and were included in the analysis (Figure 1). The median age of the study population was 33 years (range 19–82), and 234 (63.2%) were women. The median length of stay abroad was 21 days (range 6–90 days). The most common reason for travel was vacation (n = 277).
Of the 370 participants, 113 (30.5%) whose pretravel swab samples were negative acquired MDR-E during foreign travel. Of these 113 participants, 19 (16.8%) still carried MDR-E 6 months after return. In 32 of the 370 participants (8.6%), MDR-E was identified before travel. Twenty (62.5%) of these 32 participants returned with MDR-E, 7 (35.0%) of whom were still colonized after 6 months. No MDR-E was found before or after travel in 225 (60.8%) participants.
Travel-associated Risk Factors for ESBL Acquisition in Returning Travelers
For the analysis of travel-associated risk factors, data for 338 participating returning travelers with negative pretravel rectal swab sample test results were used (Technical Appendix Table 1). In total, 65 countries were visited; these are subdivided in 10 subcontinents. The most common destinations were Indonesia (n = 62), Thailand (n = 30), Malaysia (n = 27), Cambodia (n = 21), People’s Republic of China (n = 39), Kenya (n = 30), Tanzania (n = 24), Surinam (n = 20), and South Africa (n = 19).
The highest ESBL-E acquisition rates were identified among participants who visited countries in Asia: 73% in South Asia and 67% in East Asia. Univariate and multivariate analyses showed that the travel destinations South and East Asia were significant risk factors for the acquisition of ESBL-E (p<0.001). Participants traveling to Asia (all subcontinents) were more likely to return with ESBL-E colonization after a self-arranged trip (odds ratio 1.7; p = 0.07) or if they stayed in hostels/lodges (odds ratio 1.9; p = 0.08), although this finding was not statistically significant. There were no other risk factors for the acquisition of ESBL-E after foreign travel. The incidence proportions of ESBL-E after foreign travel are listed in Table 1.
Microbiological Results and Molecular Characterization
A total of 133 participants were colonized with MDR-E after travel. This group consisted of 113 travelers who had initially negative pretravel swab samples. In addition, 20 participants who had positive pretravel samples also returned colonized with MDR-E. The ESBL-E of these 133 post-travel swab samples consisted of 146 E. coli, 10 K. pneumoniae, and 2 Enterobacter cloacae isolates.
No carbapenemase-producing MDR-E were found among the pre-and post-travel isolates. Molecular characterization of the post-travel isolates demonstrated that CTX-M group 1 ESBL (n = 110) predominated (CTX-M-1–like, n = 4; CTX-M-3–like, n = 1; CTX-M-15–like, n = 85; CTX-M-32–like, n = 20), followed by CTX-M group 9 ESBL (n = 42), CTX-M group 2 (n = 2), and CTX-M group8/25 (n = 1). One E. coli isolate carried an SHV-ESBL (238S+240K). In addition, some isolates coproduced plasmid-mediated AmpC β-lactamase, ACT/MIR (n = 1) or CMY-2 (n = 2).
Thirty-four ESBL-E were isolated from pretravel rectal swab samples from 32 participants: 29 (85.3%) samples were positive for E. coli, 4 for K. pneumoniae (11.8%), and 1 for Citrobacter freundii (2.9%). The CTX-M group 1 ESBL (n = 22) comprised (CTX-M–1 like, n = 4; CTX-M-15–like, n = 16; CTX-M-32–like, n = 2); the remaining ESBL isolates belonged to CTX-M group 9 ESBL (n = 8) and CTX-M group 2 (n = 1). Two E. coli isolates carried an SHV-ESBL (238S+240K).
Coresistance to other classes of antimicrobial drugs was common in pre- and post-travel isolates; 67% displayed resistance to trimethoprim/sulfamethoxazole, 36% to ciprofloxacin, 37% to tobramycin, 35% to gentamicin, and 29% to nitrofurantoin. All isolates were susceptible to colistin and carbapenems.
MLST of ESBL-producing E. coli Isolates
MLST of 146 E. coli isolates from the post-travel samples identified 86 different STs; 31 new STs were found. The most prevalent STs were: ST38 (12%; n = 17), ST10 (7%; n = 10), and ST131 (4%; n = 9). The distribution of the CTX-M groups and types and STs is displayed in Figure 2. There was no association between ST and ESBL type, nor were STs associated with specific travel destinations. Pretravel isolates showed a similar diversity of STs, of which 3 were ST131.
Prolonged Carriage and Household Contacts
Of the 133 participants whose samples were positive for ESBL-E after return, 127 (95.4%) completed the follow-up survey and provided samples after 6 months. ESBL-E was isolated from 26 (20.4%) samples (Table 2). None of these participants reported the use of antimicrobial drugs or were hospitalized during the previous 6 months; none were health care workers, and none reported contact with farm animals. Diarrhea was reported by 7 participants.
Of 113 participants who had initially negative pretravel samples and positive samples immediately after return, 19 (16.8%) were still colonized after 6 months. Of these, 7 participants had samples that were positive for E. coli with the same ST 6 months after return. Nine participants were positive for E. coli and had a different ST 6 months after return; 3 were positive for a different species 6 months after return. Eleven household contacts of 4 MDR-E–positive participants agreed to cooperate and submitted a rectal swab sample. ESBL-producing E. coli was isolated from 2 (18.1%) household contacts, each from different households. The first household contact carried a different ESBL-producing E. coli than the associated traveler before and after the trip. Both isolates carried a CTX-M group 9 enzyme. The second household contact was positive for SHV-ESBL-producing E. coli ST2599. The associated traveler’s samples were positive for E. coli ST617 and ST38 immediately after the trip, K. pneumoniae 6 months after return, and the fourth rectal swab sample was positive for a CTX-M-15–like E. coli ST3363.
Of 20 participants whose samples were positive before and after return, 7 (35.0%) participants were still colonized 6 months after return. Of these 7 participants, 5 carried a similar strain: 2 carried a CTX-M group 9–producing E. coli with an identical ST as before the trip, 2 carried a similar ST but with a different CTX-M group enzyme as before the trip, and 1 participant carried a CTX-M group 1–producing K. pneumoniae during the study period; 2 participants returned with E. coli with a different ST. No household contacts were included in this subgroup of travelers.
The results of this study show a high ESBL-E carriage rate of 30.5% among healthy participating travelers from the Netherlands after return. This finding is worrisome, because this ESBL-E carriage rate is higher compared with those in recent studies that identified international travel as an independent risk factor for ESBL-E colonization (1–4). It is striking that none of the potential travel-associated risk factors investigated in this study, other than traveling to South and East Asia, were found to contribute to this high ESBL-E carriage rate. Additional risk factors were not revealed by including in the univariate analysis the 13 participants who had a positive pretravel sample and acquired an ESBL-producing E. coli during travel with a different ST than before the trip.
Tangden et al. associated gastroenteritis during travel with the risk for ESBL-E acquisition among travelers from Sweden (3). That association was not found in this study, which may reflect less fecal–oral contamination while traveling. Baaten et al. reported that diseases transmitted by the fecal–oral route among travelers to nonindustrialized countries have declined because of improved hygiene standards at the destination as measured by the human developmental index, sanitation index, and the water source index (8). The sanitation index levels, which represent the proportion of the population that has access to sanitation, were the lowest for sub-Saharan Africa and the Indian subcontinent. On the basis of these indices, we would expect the incidence of ESBL-E acquisition to be similar among travelers in countries in Asia and Africa. Nonetheless, participating travelers to Asia had the highest post-travel colonization rates. Travelers to Asia most likely differ in their eating habits compared with travelers to African countries, since the former are more likely to eat in individual establishments outside of hotels or from street vendors. Thus, the high incidence rate found for returning travelers from Asia in this study may result from the increased risk for foodborne exposure.
No CP-E were found despite the fact that countries were visited where CP-E are prevalent in hospitals and in the environment (5). Other known risk areas besides India for the acquisition of CP-E, such as the United States, Greece, Italy, and the Balkan region were not included in this study, because these travelers do not visit the Travel Clinic of the Leiden University Medical Center. Many citizens from the Netherlands have relatives in North African countries or Turkey whom they visit frequently. OXA-48–producing bacteria are endemic to these countries (9). These travelers do not consult travel clinics and may well return carrying OXA-48–producing isolates unnoticed.
Peirano et. al. (2) reported that the prevalence of ST131, a uropathogenic E. coli notorious for its worldwide expansion and spread of CTX-M-15, was similar among travelers and non-travelers from the Calgary region. The most prevalent ESBL among the travelers participating in this study was the CTX-M-15–like enzyme. However, this enzyme was found in a plethora of different STs of E. coli. Participants in the Leiden area not only showed a great heterogeneity of STs but also harbored different CTX-M types after travel and 6 months after return. The majority of the E. coli strains identified in the participants in this study were of STs that clustered around ST10 and belonged to ST complex 10 (STC10). STC10 strains essentially belong to the nonvirulent, commensal phylogenetic group A (10). In a recent study based in France, isolates belonging to STC10 were found to be the most prevalent among fecal samples from healthy carriers of nalidixic acid–resistant (but ESBL-negative) E. coli (11). It is also the most prevalent STC in the MLST database. Data from this study show that transmissible genetic elements containing resistance genes are exchanged with naive E. coli strains of the human intestinal microbiota during foreign travel combined with foodborne exposure.
Although 26 participants had positive results for ESBL-E 6 months after travel, they were not all positive for the same enterobacterial strain that was identified immediately after travel. In 8 participating travelers colonized with E. coli, an ESBL of the same CTX-M group was identified in the immediate post-travel sample as after 6 months, but E. coli with a different ST was detected. In 11 travelers, the strain persisted during the study period. It is possible that more strain types were present in the rectal samples where colony morphology of different strains was not discriminative. However, it is also possible that the transfer of ESBL genes between strains within a host is a frequent occurrence. Or, the acquisition of a new ESBL-E occurs at the expense of the resident strain.
Interhousehold transmission of ESBL-E has been demonstrated in the community setting (12,13). Clonally related strains could be found for 66% of the isolates from infected community patients and their corresponding household contacts (13). Because of the limited data on household contacts in the present study, the transmission dynamics of ESBL-E in households after foreign travel remain to be discovered.
The high pretravel ESBL-E carriage rate among our study participants (8.6%) was an unexpected finding. Two recent studies on the ESBL-E carriage rate in the community have been conducted in the Amsterdam area. In the first study, 10.1% of the fecal samples from outpatients with gastrointestinal discomfort being assessed by their general practitioners yielded ESBL-E, predominantly CTX-M-15–producing E. coli (14). In a second study, investigating the prevalence of ESBL-E carriage in the general community, a carriage rate of 8.5% was found (E.A. Reuland et al., unpub. data). Although no data on travel history were given, the investigators pointed out that foreign travel might be responsible for at least part of ESBL-E carriage rates among outpatients from the Netherlands. This finding is supported by data from our study: 50% of participants who had a positive pretravel sample had traveled during the previous 12 months. This high percentage of carriers identified in this study before travel points toward ongoing importation of ESBL-E. Other potential reservoirs for ESBL-E are poultry and other retail meats, which have been found to be contaminated with ESBL-producing E. coli strains harboring the genes on identical plasmids as found in human isolates (15,16).
International travel is growing and the number of intercontinental flights has increased during the past decade. The findings in this study support the role of international travel on the ESBL-E acquisition and carriage rates among travelers from the Netherlands, especially to South and East Asia. The high pre- and post–travel carriage rates among persons traveling from the Netherlands indicate that the consequences of increased foreign travel are already manifest in this country. The lack of apparent travel-associated risk factors, the spread of CTX-M enzymes through a highly diverse population of E. coli, the association of ESBL production with multidrug resistance, and the possible role of other sources make containing the spread difficult. These factors also complicate the implementation of other strategies, such as pretravel advice, and imply that all travelers to Asia should be considered for carriage of ESBL-E. Although CP-E were not found in this study, CP-E have been introduced into the Netherlands by returning travelers (6,17–19), and introduction by asymptomatic travelers to the Netherlands from countries where CP-E are endemic may largely go unnoticed. There is no reason to assume that, after CP-E are introduced, their spread will be less dynamic than that of ESBL-E. This inference has serious implications for the implementation of screening methods and effective infection control strategies. On the basis of the results of this study, we recommend active surveillance of CP-E and ESBL-E and at least temporary contact isolation precautions for patients being admitted to hospitals after travel to Asia during the previous 6 months.
Dr Paltansing is a clinical microbiologist in the Department of Medical Microbiology at the Leiden University Medical Center, the Netherlands. Her research interests include the molecular characterization and epidemiology of MDR-E.
Acknowledgments
We thank the technicians from the Department of Medical Microbiology for performing the cultures used in this study and Check-Points B.V. for technical support.
This study was partly funded by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID SG Research Grant) and by the Department of Medical Microbiology of the Leiden University Medical Center, the Netherlands.
References
- Kennedy K, Collignon P. Colonisation with Escherichia coli resistant to “critically important” antibiotics: a high risk for international travellers. Eur J Clin Microbiol Infect Dis. 2010;29:1501–6 . DOIPubMedGoogle Scholar
- Peirano G, Laupland KB, Gregson DB, Pitout JD. Colonization of returning travelers with CTX-M–producing Escherichia coli. J Travel Med. 2011;18:299–303. DOIPubMedGoogle Scholar
- Tängdén T, Kars O, Mehus A, Lowdin E. Foreign travel is a major risk factor for colonization with Escherichia coli producing CTX-M–type extended-spectrum beta-lactamases: a prospective study with Swedish volunteers. Antimicrob Agents Chemother. 2010;54:3564–8 . DOIPubMedGoogle Scholar
- Weisenberg SA, Mediavilla JR, Chen L, Alexander EL, Rhee KY, Kreiswirth BN, Extended spectrum beta-lactamase–producing Enterobacteriaceae in international travelers and non-travelers in New York city. PLoS ONE. 2012;7:e45141. DOIPubMedGoogle Scholar
- van der Bij AK, Pitout JD. The role of international travel in the worldwide spread of multiresistant Enterobacteriaceae. J Antimicrob Chemother. 2012;67:2090–100. DOIPubMedGoogle Scholar
- Leverstein-van Hall MA, Stuart JC, Voets GM, Versteeg D, Roelofsen E, Fluit AC. Carbapenem-resistant Klebsiella pneumoniae following foreign travel [in Dutch]. Ned Tijdschr Geneeskd. 2010;154:A2013 .PubMedGoogle Scholar
- Cohen Stuart J, Dierikx C, Al-Naimi NN, Karczmarek A, Van Hoek H, Vos P, Rapid detection of TEM, SHV and CTX-M extended-spectrum beta-lactamases in Enterobacteriaceae using ligation-mediated amplification with microarray analysis. J Antimicrob Chemother. 2010;65:1377–81. DOIPubMedGoogle Scholar
- Baaten GG, Sonder GJ, Van Der Loeff MF, Coutinho RA, Van Den Hoek A. Fecal-orally transmitted diseases among travelers are decreasing due to better hygienic standards at travel destination. J Travel Med. 2010;17:322–8. DOIPubMedGoogle Scholar
- Nordmann P, Naas T, Poirel L. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis. 2011;17:1791–8 . DOIPubMedGoogle Scholar
- Bengtsson S, Naseer U, Sundsfjord A, Kahlmeter G, Sundqvist M. Sequence types and plasmid carriage of uropathogenic Escherichia coli devoid of phenotypically detectable resistance. J Antimicrob Chemother. 2012;67:69–73. DOIPubMedGoogle Scholar
- Leflon-Guibout V, Blanco J, Amaqdouf K, Mora A, Guize L, Nicolas-Chanoine MH. Absence of CTX-M enzymes but high prevalence of clones, including clone ST131, among fecal Escherichia coli isolates from healthy subjects living in the area of Paris, France. J Clin Microbiol. 2008;46:3900–5. DOIPubMedGoogle Scholar
- Lo WU, Ho PL, Chow KH, Lai EL, Yeung F, Chiu SS. Fecal carriage of CTXM type extended-spectrum beta-lactamase–producing organisms by children and their household contacts. J Infect. 2010;60:286–92. DOIPubMedGoogle Scholar
- Valverde A, Grill F, Coque TM, Pintado V, Baquero F, Canton R, High rate of intestinal colonization with extended-spectrum-beta-lactamase–producing organisms in household contacts of infected community patients. J Clin Microbiol. 2008;46:2796–9. DOIPubMedGoogle Scholar
- Reuland EA, Overdevest IT, Al-Naimi NN, Kalpoe JS, Rijnsburger MC, Raadsen SA, High prevalence of ESBL-producing Enterobacteriaceae carriage in Dutch community patients with gastrointestinal complaints. Clin Microbiol Infect. 2013;19:542–9. DOIPubMedGoogle Scholar
- Kluytmans JA, Overdevest IT, Willemsen I, Kluytmans-van den Bergh MF, Van der Zwaluw K, Heck M, Extended-spectrum beta-lactamase–producing Escherichia coli from retail chicken meat and humans: comparison of strains, plasmids, resistance genes, and virulence factors. Clin Infect Dis. 2013;56:478–87. DOIPubMedGoogle Scholar
- Overdevest I, Willemsen I, Rijnsburger MC, Eustace A, Xu L, Hawkey P, Extended-spectrum beta-lactamase genes of Escherichia coli in chicken meat and humans, the Netherlands. Emerg Infect Dis. 2011;17:1216–22. DOIPubMedGoogle Scholar
- Halaby T, Reuland AE, Al Naimi N, Potron A, Savelkoul PH, Vandenbroucke-Grauls CM, A case of New Delhi metallo-beta-lactamase 1 (NDM-1)–producing Klebsiella pneumoniae with putative secondary transmission from the Balkan region in the Netherlands. Antimicrob Agents Chemother. 2012;56:2790–1. DOIPubMedGoogle Scholar
- Kalpoe JS, Al-Naimi NN, Poirel L, Nordmann P. Detection of an Ambler class D OXA-48-type beta-lactamase in a Klebsiella pneumoniae strain in the Netherlands. J Med Microbiol. 2011;60:677–8 . DOIPubMedGoogle Scholar
- Leverstein-Van Hall MA, Cohen Stuart J, Voets GM, Versteeg D, Tersmette T, Fluit AC. Global spread of New Delhi metallo-beta-lactamase 1. Lancet Infect Dis. 2010;10:830–1. DOIPubMedGoogle Scholar
Figures
Tables
Follow Up
Earning CME Credit
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions (with a minimum 70% passing score) and earn continuing medical education (CME) credit, please go to www.medscape.org/journal/eid. Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers. You must be a registered user on Medscape.org. If you are not registered on Medscape.org, please click on the New Users: Free Registration link on the left hand side of the website to register. Only one answer is correct for each question. Once you successfully answer all post-test questions you will be able to view and/or print your certificate. For questions regarding the content of this activity, contact the accredited provider, CME@medscape.net. For technical assistance, contact CME@webmd.net. American Medical Association’s Physician’s Recognition Award (AMA PRA) credits are accepted in the US as evidence of participation in CME activities. For further information on this award, please refer to http://www.ama-assn.org/ama/pub/category/2922.html. The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMA PRA credit may be acceptable as evidence of participation in CME activities. If you are not licensed in the US, please complete the questions online, print the certificate and present it to your national medical association for review.
Article Title:
Extended-Spectrum β-Lactamase–producing Enterobacteriaceae among Travelers from the Netherlands
CME Questions
1. You are seeing a 50-year-old woman for a routine physical exam before she embarks on a 3-month trip around the world. She is concerned regarding the possibility of illness during her travels. Based on the current study by Paltansing and colleagues, what should you consider regarding acquisition of multidrug-resistant Enterobacteriaceae (MDR-E) during travel?
A. Incident colonization occurred in approximately 80% of travelers
B. Incident colonization occurred in approximately 30% of travelers
C. MDR-E was not found among any individual prior to travel
D. Over 90% of MDR-E found after travel was carbapenemase-producing Enterobacteriaceae (CPE)
2. What can you tell this patient was the region most associated with colonization with MDR-E in the current study?
A. Eastern Europe
B. Sub-Saharan Africa
C. Asia
D. Central America
3. Which of the following was the most significant risk factor for colonization with MDR-E after returning from travel?
A. Colonization with MDR-E prior to travel
B. Older age
C. Recent antibiotic use
D. A history of diarrhea during travel
4. What else should you consider regarding the acquisition of extended-spectrum β-lactamase–producing Enterobacteriaceae (ESBL-E) during travel in the current study?
A. CTX-M enzymes were the predominant ESBLs
B. Coresistance to other antibiotics was rare among ESBL-E
C. ESBL-E was not found among any carrier after 6 months of follow-up
D. ESBL-E was not found among household contacts of carriers
Activity Evaluation
|
1. The activity supported the learning objectives. |
||||
|
Strongly Disagree |
|
|
|
Strongly Agree |
|
1 |
2 |
3 |
4 |
5 |
|
2. The material was organized clearly for learning to occur. |
||||
|
Strongly Disagree |
|
|
|
Strongly Agree |
|
1 |
2 |
3 |
4 |
5 |
|
3. The content learned from this activity will impact my practice. |
||||
|
Strongly Disagree |
|
|
|
Strongly Agree |
|
1 |
2 |
3 |
4 |
5 |
|
4. The activity was presented objectively and free of commercial bias. |
||||
|
Strongly Disagree |
|
|
|
Strongly Agree |
|
1 |
2 |
3 |
4 |
5 |
1These authors contributed equally to this article.
Related Links
Table of Contents – Volume 19, Number 8—August 2013
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Sunita Paltansing, Department of Medical Microbiology, Leiden University Medical Center, PO Box 9600, 2300 RC, Leiden, the Netherlands
Top