Volume 22, Number 9—September 2016
Letter
Inactivation and Environmental Stability of Zika Virus
To the Editor: Zika virus is an emerging virus that has spread to most countries in Latin America and the Caribbean (1,2). It is transmitted by mosquitoes and through sexual intercourse (3). Most persons infected with Zika virus are asymptomatic or experience mild symptoms (4). However, in a pregnant woman, infection may cause severe pregnancy and birth complications, most notably microcephaly in children infected in utero (5–7). Zika virus infection might also be associated with an increased incidence of Guillain-Barré syndrome (8). Thus, the virus represents a threat to healthcare workers who manage infected patients or diagnostic samples and researchers who work with infectious virus in laboratories.
Working with Zika virus, a Biosafety Level 2 (BSL-2) pathogen in the European Union, except for the United Kingdom (where it is BSL-3), requires specific safety precautions (9). No inactivation data specific for Zika virus are available (9); consequently, disinfection guidelines are based on protocols to inactivate other flaviviruses. To gain experimental evidence regarding inactivation and disinfection for Zika virus, we determined its susceptibility to various disinfectants and inactivation methods.
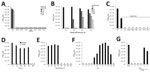
To test susceptibilities, we determined the 50% tissue cell infectious dose per milliliter (TCID50/mL) (10) of the Zika virus MR766 strain (1) before and after the virus was exposed to disinfectants or other inactivation procedures (Technical Appendix). We then determined the effect of alcohol-based disinfectants on viral infectivity. Using Zika virus stock containing 2.5% fetal calf serum (FCS) mixed 3:10 (vol/vol) with indicated alcohols, we incubated the mixture for 1 minute and then used it for infection (Figure, panel A). All alcohols entirely inactivated the virus. Complete loss of infectivity was also observed after virus exposure to 1% hypochlorite (often used to inactivate virus in liquid wastes in BSL-2/3 laboratories), 2% paraformaldehyde (used to inactivate virus for subsequent flow cytometry), and 2% glutaraldehyde (often applied to fix virus for subsequent electron microscopy analysis) (Figure, panel A). Thus, routinely used disinfectants and inactivation procedures are sufficient to inactivate Zika virus in laboratory virus stocks. Next, we repeated these experiments in the presence of a high protein load using Zika virus preparations supplemented with FCS in increasing concentrations (10%, 40%, 90%), to mimic virus found in clinically relevant material. Again, all treatments entirely disrupted Zika virus infectivity (Figure, panel A).
Ultraviolet (UV) radiation inactivates viruses by chemically modifying the genome. We exposed 200 μL of Zika virus preparations containing increasing concentrations of serum to UV light of a laminar flow for up to 60 minutes. Exposure for 10 minutes entirely inactivated Zika virus in the presence of 2.5% FCS serum; increasing concentrations of serum reduced the antiviral effects of UV light (Figure, panel B). When Zika virus containing 90% serum was exposed for 60 min to UV light, infectivity was reduced by 99.95%; however, some residual infectivity was detected (Figure, panel B).
Next, we evaluated environmental stability by drying 100 μL of Zika virus stock for 18 hours. Thereafter, dried virus was reconstituted in the same volume of medium or disinfectants. Endpoint titrations showed that the reconstituted virus remained infectious, although TCID50 was reduced by ≈3 orders of magnitude (Figure, panel C). All disinfectants and UV radiation entirely inactivated dried Zika virus (Figure, panel C). Additional experiments demonstrated that dried Zika virus remained infectious for >3 days (Figure, panel D) suggesting, for example, that dried droplets can be infectious, confirming that proper surface disinfection is essential.
We also assessed the environmental stability of Zika virus to heat and change in pH. The virus was stable at temperatures up to 50°C but lost all infectivity at temperatures of >60°C (Figure, panel E). Thus, virus-contaminated materials such as surgical instruments can be decontaminated by heat. We also found that Zika virus infectivity was highest after adjusting the stock to a pH of ≈9 (Figure, panel F). In contrast, adjusting Zika virus to pH 12 or to <pH 4 abrogated infectivity (Figure, panel F).
Finally, we analyzed whether gloves routinely used in BSL-2 laboratories protect against Zika virus. For this, we cut off fingertips of nitrile and latex gloves, filled tips with a Zika virus suspension, and placed them into cell culture plates containing medium. Virus-containing fingertips were inserted in such a way that diffusion would only occur if the virus passed through the nitrile/latex barrier. As a control, we made a hole of <1 mm in the fingertips. All 3 tested gloves prevented virus diffusion (Figure, panel G). However, if glove integrity was disrupted by a pin, the virus passed through in 2 of 3 cases (Figure, panel G).
We demonstrated that Zika virus is destroyed by classical disinfectants and inactivation methods and that nitrile and latex gloves are protective. We also showed that UV light of a laminar flow hood inactivates Zika virus, but particularly if the virus is in a protein-rich environment, the exposure time range should be in hours rather than in minutes. Although we expected that Zika virus would be inactivated by alcohol and disinfectants, we conducted a thorough experimental verification to exclude uncertainties surrounding work with this emerging pathogen.
References
- Dick GWA, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46:509–20. DOIPubMedGoogle Scholar
- Zammarchi L, Tappe D, Fortuna C, Remoli ME, Günther S, Venturi G, Zika virus infection in a traveller returning to Europe from Brazil, March 2015. Euro Surveill. 2015;20:21153. DOIPubMedGoogle Scholar
- D’Ortenzio E, Matheron S, Yazdanpanah Y, de Lamballerie X, Hubert B, Piorkowski G, Evidence of sexual transmission of Zika virus. N Engl J Med. 2016;374:2195–8. DOIPubMedGoogle Scholar
- Ginier M, Neumayr A, Günther S, Zika without symptoms in returning travellers: what are the implications? Travel Med Infect Dis. 2016;14:16–20. DOIPubMedGoogle Scholar
- Driggers RW, Ho C-Y, Korhonen EM. Kuivanen S1, Jääskeläinen AJ1, Smura T, et al. Zika virus infection with prolonged maternal viremia and fetal brain abnormalities. N Engl J Med. 2016;374:2142–51. DOIPubMedGoogle Scholar
- Rasmussen SA, Jamieson DJ, Honein MA, Zika virus and birth defects—reviewing the evidence for causality. N Engl J Med. 2016;374:1981–7. DOIPubMedGoogle Scholar
- Mlakar J, Korva M, Tul N, Petersen LR, Zika virus associated with microcephaly. N Engl J Med. 2016;374:951–8. DOIPubMedGoogle Scholar
- Rozé B, Najioullah F, Fergé J-L, Apetse K, Brouste Y, Cesaire R, Zika virus detection in urine from patients with Guillain-Barré syndrome on Martinique, January 2016. Euro Surveill. 2016;21:30154. DOIPubMedGoogle Scholar
- Charrel RN, Leparc-Goffart I, Pas S, de Lamballerie X, Koopmans M, Reusken C. State of knowledge on Zika virus for an adequate laboratory response. Bull World Health Organ. E-pub 2016 Feb 10. DOIGoogle Scholar
- Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–7.
Figure
Cite This ArticleRelated Links
Table of Contents – Volume 22, Number 9—September 2016
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Jan Münch, Institute of Molecular Virology, Ulm University Medical Centre, Meyerhofstrasse 1, 89081 Ulm, Germany
Top