Volume 23, Number 6—June 2017
Dispatch
High Rates of Neutralizing Antibodies to Toscana and Sandfly Fever Sicilian Viruses in Livestock, Kosovo
Abstract
Toscana and sandfly fever Sicilian viruses (TOSV and SFSV, respectively), both transmitted by sand flies, are prominent human pathogens in the Old World. Of 1,086 serum samples collected from cattle and sheep during 2013 in various regions of Kosovo (Balkan Peninsula), 4.7% and 53.4% had neutralizing antibodies against TOSV and SFSV, respectively.
Phleboviruses (family Bunyaviridae, genus Phlebovirus) are negative-sense tri-segmented RNA viruses for which mosquitoes, ticks, and sand flies are vectors. In the Old World, phleboviruses transmitted by sand flies (phlebotomines) are expanding in the Mediterranean basin, where an increasing number of new viruses have been identified (1). There, sand fly–borne phleboviruses are divided into 3 groups in accordance with their antigenic relationships. Two groups correspond to recognized species: Sandfly fever Naples virus (including sandfly fever Naples [SFNV], Massilia, Tehran, and Toscana [TOSV] viruses) and Salehabad virus (including Salehabad and Arbia viruses). The third group comprises 2 viruses classified as tentative species: Sandfly fever Sicilian virus (SFSV) and Corfou virus (2). Several are historic human pathogens, such as SFSV and SFNV, which cause sandfly fever syndrome, a self-limited but severely incapacitating febrile illness (1); TOSV can cause central and peripheral nervous system infections, such as meningitis and encephalitis (3).
Although first data on sandfly fever were acquired from the Balkan region, few studies were published specifically about the situation in Kosovo (4): in 1976, a total of 9.6% of human serum samples contained neutralizing antibodies against SFSV (5) (the exact region of Kosovo was not mentioned), and in 2011, <1% of human serum samples collected in the Pejë region contained TOSV neutralizing antibodies (6). Neutralizing antibody–based seroprevalence studies using animal serum proved interesting regarding the global circulation of corresponding viruses, as recently described in Portugal, Tunisia, and Greece (7–9). To improve understanding of the circulation of TOSV and SFSV in Kosovo, we tested serum samples collected in cattle and sheep through neutralizing assay and field-trapped sand flies for viral RNA.
In 2013, serum from domestic animals was collected from 12 different regions of Kosovo. Samples were collected from 933 cattle and 153 sheep from 9 and 5 different regions, respectively, in Kosovo; the information (location, specimen, date) were recorded. Ten milliliters of blood was taken from jugular venipuncture, and serum was separated by centrifugation. All samples were stored at −20°C.
We tested cattle and sheep serum for neutralizing antibodies using the virus microneutralization assay, described for phleboviruses (7) in parallel with TOSV strain MRS2010-4319501 and SFSV strain Sabin. Serum samples were diluted from 1:10 to 1:80 into 96-well plates with a volume of 50 μL. Except for controls, we added a 1,000 50% tissue culture infective dose in a 50-μL volume. For controls, we added 50 μL of Eagle's minimum essential medium enriched with 5% fetal bovine serum, 1% penicillin–streptomycin, 1% L-glutamine 200 mmol/L, 1% kanamycin, and 3% fungizone. The plates were incubated at 37°C. After 1 h, a 100 μL suspension of 2 × 105 Vero cells/mL was added and incubated at 37°C in the presence of 5% CO2. The microplates were read under an inverted microscope after 5 days for TOSV and 6 days for SFSV, and the presence (neutralization titer at 10, 20, 40, and 80) or absence (no neutralization) of cytopathic effect was noted. Cutoff value for positivity was set at titer >20 (8).
In 2014, a total of 267 sand flies were trapped and identified. We tested these sand flies for phleboviruses using previously described protocols (9).
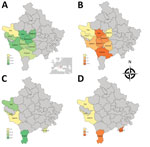
Global rates of TOSV neutralizing antibodies were in the same magnitude in cattle (5.14%) and sheep (1.96%) in Kosovo (Table; Figure). Results observed in Pejë were congruent with recent findings obtained with human serum; in both cases, TOSV circulation appears limited (6). Although SFNV and TOSV belong to the same serocomplex, they can be distinguished by using neutralization; therefore, the high rate (27.9%) of SFNV neutralizing antibodies in humans reported in the 1970s (5) most likely reflects circulation of SFNV rather than TOSV, a finding that did not differ from our results and recent results reported by others (6). Rates of TOSV neutralizing antibodies were highest in southwestern regions of Kosovo, whereas negative results were obtained in the Pejë area (Pejë, Deçan, Junik). Although we did not detect viral RNA in the 267 tested sand flies, TOSV was reported in Croatia and Greece (10,), and a new phlebovirus was described in Albania, Croatia, and Bosnia-Herzegovina (9; N. Ayhan et al., unpub. data).
Rates of SFSV neutralizing antibodies were much higher than those for TOSV. Results for cattle ranged from 24.0% to 78.2% (mean 58.5%); results for sheep were lower, ranging from 2.7% to 56.7% (mean 22.2%). For sheep, 4 of the 5 regions had rates of 14.0%–56.7%; the rate was much lower (2.7%) in Gjakovë.
Few data are available for comparison; 9.6% of tested human serum contained SFSV neutralizing antibodies in the 1970s (5). Although no direct evidence (molecular detection of viral RNA or virus isolation) exists of SFSV or another SFSV-like virus in Kosovo or neighboring countries, our results imply the presence of either SFSV or an SFSV-related virus in Kosovo. We consider it valid to use SFSV as a surrogate for all SFSV-related viruses (sandfly fever Turkey virus, sandfly fever Cyprus virus) because amino acid distances observed between the proteins that elicit neutralizing antibodies (Gn and Gc) are well within the acceptable range, (i.e., <5% different for SFSV and SFSV-related viruses) (7). Thus, neutralizing antibodies are unlikely to discriminate between closely related SFSV isolates.
Recent seroprevalence studies showed high seroprevalence rates for SFSV neutralizing antibodies in dogs in Portugal (50.8%) (7), Tunisia (38.1%–59.2% depending on the region) (8), and Greece (71.9%) and Cyprus (60.2%) (11). Our results are congruent with data from continental Greece, with rates in the same order of magnitude (12). All these findings verify the high prevalence of SFSV in the Mediterranean basin. Because of the nature of this study, the serum was collected and stored under conditions that prevented attempts to detect viral RNA and isolate viral strains. SFSV remains an important human pathogen, as recently highlighted in Africa and Turkey (12,13).
Published data about the distribution of sand flies and species identification are old and scarce; Phlebotomus papatasi, P. perfiliewi, P. neglectus, and P. tobbi sand flies have been documented in Kosovo and neighboring countries with similar environmental and climatic conditions (14,15). In our study, P. major sand flies dominated (81%), followed by P. tobbi (11.2%), P. simici (3.7%), P. papatasi (1%), and other species (3.1%).
SFSV positivity varied among the regions for cattle and sheep. The regional prevalence differences might have resulted from geographic and climatic characteristics of the region that could affect the vector sand fly species distribution and population size.
Our results confirm that TOSV and SFSV, or an SFSV-like virus, are circulating in several regions of Kosovo, which indicates that humans are exposed to these viruses. This finding merits confirmation through seroprevalence studies and initiation of systematic testing for TOSV and SFSV real-time reverse transcription PCR for febrile illness and central nervous system infections during the warm season.
Ms. Ayhan is a PhD student at Aix Marseille University. Her research interests include phleboviruses transmitted by sand flies in the Old World.
Acknowledgment
This work was supported in part by the European Virus Archive Goes Global project, which has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement no. 653316; the EDENext FP7-no. 261504 European Union project and this paper is catalogued by the EDENext Steering Committee as EDENext469 (http://www.edenext.eu). The work of N.A. and R.N.C. was conducted under the frame of EurNegVec COST Action TD1303. N.A. is supported by the Fondation Mediterranee Infection.
References
- Alkan C, Bichaud L, de Lamballerie X, Alten B, Gould EA, Charrel RN. Sandfly-borne phleboviruses of Eurasia and Africa: epidemiology, genetic diversity, geographic range, control measures. Antiviral Res. 2013;100:54–74. DOIPubMedGoogle Scholar
- Plyusnin A, Beaty BJ, Elliott RM, Goldbach R, Kormelink R, Lundkvist A, et al. Bunyaviridae. In: King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ, editors. Virus taxonomy: classification and nomenclature of viruses. Ninth report of the International Committee on Taxonomy of Viruses. San Diego (CA): Elsevier; 2011. p. 693–709.
- Charrel RN, Gallian P, Navarro-Mari JM, Nicoletti L, Papa A, Sánchez-Seco MP, et al. Emergence of Toscana virus in Europe. Emerg Infect Dis. 2005;11:1657–63. DOIPubMedGoogle Scholar
- Pick A. Zur pathologie und therapie einer eigenthumlichen endemischen Krankheitsform. Wien Med Wochenschr. 1886;33:1141–5.
- Tesh RB, Saidi S, Gajdamovič SJA, Rodhain F, Vesenjak-Hirjan J. Serological studies on the epidemiology of sandfly fever in the Old World. Bull World Health Organ. 1976;54:663–74.PubMedGoogle Scholar
- Venturi G, Marchi A, Fiorentini C, Ramadani N, Quaglio G, Kalaveshi A, et al. Prevalence of antibodies to phleboviruses and flaviviruses in Peja, Kosovo. Clin Microbiol Infect. 2011;17:1180–2. DOIPubMedGoogle Scholar
- Alwassouf S, Maia C, Ayhan N, Coimbra M, Cristovao JM, Richet H, et al. Neutralization-based seroprevalence of Toscana virus and sandfly fever Sicilian virus in dogs and cats from Portugal. J Gen Virol. 2016;97:2816–23. DOIPubMedGoogle Scholar
- Sakhria S, Alwassouf S, Fares W, Bichaud L, Dachraoui K, Alkan C, et al. Presence of sandfly-borne phleboviruses of two antigenic complexes (Sandfly fever Naples virus and Sandfly fever Sicilian virus) in two different bio-geographical regions of Tunisia demonstrated by a microneutralisation-based seroprevalence study in dogs. Parasit Vectors. 2014;7:476.PubMedGoogle Scholar
- Ayhan N, Velo E, de Lamballerie X, Kota M, Kadriaj P, Ozbel Y, et al. Detection of Leishmania infantum and a novel phlebovirus (Balkan virus) from sand flies in Albania. Vector Borne Zoonotic Dis. 2016;16:802–6. DOIPubMedGoogle Scholar
- Papa A, Paraforou T, Papakonstantinou I, Pagdatoglou K, Kontana A, Koukoubani T. Severe encephalitis caused by Toscana virus, Greece. Emerg Infect Dis. 2014;20:1417–9. DOIPubMedGoogle Scholar
- Alwassouf S, Christodoulou V, Bichaud L, Ntais P, Mazeris A, Antoniou M, et al. Seroprevalence of sandfly-borne phleboviruses belonging to three serocomplexes (sandfly fever Naples, sandfly fever Sicilian and Salehabad) in dogs from Greece and Cyprus using neutralization test. PLoS Negl Trop Dis. 2016;10:e0005063. DOIPubMedGoogle Scholar
- Ergunay K, Ayhan N, Charrel RN. Novel and emergent sandfly-borne phleboviruses in Asia Minor: a systematic review. Rev Med Virol. 2017;27:e1898. DOIPubMedGoogle Scholar
- Woyessa AB, Omballa V, Wang D, Lambert A, Waiboci L, Ayele W, et al. An outbreak of acute febrile illness caused by Sandfly Fever Sicilian Virus in the Afar region of Ethiopia, 2011. Am J Trop Med Hyg. 2014;91:1250–3. DOIPubMedGoogle Scholar
- Simitch T, Zivkovitch V. [Phlebotome fauna in Yugoslavia and their role in the epidemiology of pappataci fever, kala-azar and cutaneous leishmaniasis] [in French]. Arch Inst Pasteur Alger. 1956;34:380–7.PubMedGoogle Scholar
- Živković V. Faunistic and ecological investigation of sandflies (Diptera, Phlebotomidae) in Serbia. Acta Vet (Beogr). 1980;30:67–88.
Figure
Table
Cite This Article1These authors contributed equally to this article.
Table of Contents – Volume 23, Number 6—June 2017
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Rémi N. Charrel, Aix Marseille University, UMR_D 190 Emergence des Pathologies Virales, Faculty of Medicine, 27 blvd Jean Moulin, Marseille 13385, France
Top