Volume 25, Number 3—March 2019
Dispatch
Response to Isoniazid-Resistant Tuberculosis in Homeless Shelters, Georgia, USA, 2015–2017
Cite This Article
Citation for Media
Abstract
In 2008, an outbreak of isoniazid-resistant tuberculosis was identified among residents of homeless shelters in Atlanta, Georgia, USA. When initial control efforts involving standard targeted testing failed, a comprehensive approach that involved all providers of services for the homeless successfully interrupted the outbreak.
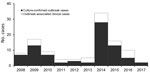
In 2008, the Tuberculosis Prevention and Control Program at the Georgia Department of Public Health (Atlanta, GA, USA) was notified of 7 cases of active tuberculosis (TB) in Fulton County, Georgia, for which isolate genotypes matched. All cases were linked to 1 homeless shelter in Atlanta, and all isolates exhibited low-level isoniazid resistance (resistant at 0.1 μg/mL but susceptible at 0.4 μg/mL according to BACTEC MGIT [Becton, Dickson and Company, https://www.bd.com]) (1). Additional cases with the same genotype were subsequently discovered at other locations. As of December 2017, a total of 110 outbreak-related cases had been identified in Fulton County (Figure 1), an additional 17 cases in other Georgia counties, and 47 cases in 15 other states. Of the 110 Fulton County cases, 41 (37%) patients were co-infected with HIV. A previous report of the outbreak through December 2015 identified several challenges associated with confronting the outbreak, notably lack of accurate rosters for overnight residents in most shelters, lack of voluntary participation in the episodic large-scale screenings (diagnostic testing with a tuberculin skin test [TST] or interferon-γ release assay, or a symptom screening for persons known to have a prior positive diagnostic test result) conducted at the shelters, and lack of consistent TB screening procedures at any of the facilities (1). An additional complication was ongoing litigation and efforts by nearby businesses and agencies of the Atlanta city government to close the index shelter (2). As a result of these issues, implementation of true targeted testing was initially limited, but in response to the large increase in the number of persons with a diagnosis of active TB caused by the outbreak strain in 2014 (Figure 1), the health department intensified its efforts. We report the results of the outbreak response in Fulton County, conducted from January 2015 through December 2017, illustrating several challenges caused by disjointed healthcare received by persons experiencing homelessness.
In June 2014, the Fulton County Board of Health joined with Mercy Care, a local federally qualified health center, to create the Metro Atlanta TB Task Force, including representatives from public health, homeless shelters, and other community service providers to provide a coordinated response in which testing targeted the entire population of persons experiencing homelessness instead of individual facilities. Before the task force interventions, none of the county’s 6 homeless shelters had adopted effective TB control guidelines (1). Furthermore, a formal survey of shelter employees revealed that their knowledge of TB disease and its transmission was low, a situation further exacerbated by rapid staff turnover. To address these issues, the task force began a series of meetings, led by the health department and Mercy Care, to develop and implement a set of comprehensive TB control guidelines for homeless shelters.
A key feature of these interventions was mandatory TB screening of shelter residents within 7 days of shelter entry and every 6 months thereafter. In addition, the guidelines included the following principles: appointment of a TB liaison at each of the 6 shelters, isolation or separation of residents with cough, maintenance of a bed log that includes every resident’s name and sleeping location within the facility, provision of a TB clearance card at the time each person completed screening to allow them access to the 6 shelters, and random inspections of the shelters by the health department to ensure adherence to the guidelines and to provide ongoing staff education. Because publication of the guidelines did not lead to their immediate uptake at all 6 shelters, educational initiatives were undertaken to increase TB knowledge and awareness of guideline content among shelter staff and the homeless community (3).
During the outbreak, persons with a diagnosis of latent TB infection were assumed to be infected with the isoniazid-resistant strain and were therefore offered daily treatment with rifampin for 4 months (4). Directly observed therapy was provided for most shelter residents starting in 2008 and for all residents after 2015; incentives ($5 coupons for local grocery stores) were offered weekly for successful completion of all doses.
Data from testing performed before 2015 showed that ≈30% of persons tested by TST did not return for reading of the results. To overcome this problem, starting in 2015, the TST was replaced as the primary test by a single-step blood test, an interferon γ-release assay (QuantiFERON Gold In-Tube; QIAGEN, https://www.qiagen.com).
From January 2015 through December 2017, a total of 14,496 persons were screened for latent TB infection, compared with 2,451 from January 2008 through December 2014 (Figure 2). During 2015–2017, a total of 3 cases of active TB were identified through screening, resulting in disease incidence of 20.7 cases/100,000 persons screened. From January 2, 2015, through December 31, 2017, a total of 430 persons received a diagnosis of untreated latent TB and 391 (91%) started treatment; of those starting treatment, 207 (53%) completed a full course. In 2017, only 2 cases of outbreak-associated TB were reported (Figure 1).
Despite an ongoing downward trend of TB in the United States (5), outbreaks among persons with a history of homelessness continue to occur regularly (6–12). As previously reported, the mobility of persons experiencing homelessness rendered standard contact tracing activities in this investigation difficult (13). Greater success was achieved with a systematic approach targeting all shelter residents for testing rather than just individual facilities in which cases had been identified. In particular, the number of persons tested and provided treatment for latent TB markedly increased after introduction of comprehensive standard screening guidelines and implementation at all shelters.
Since 2015, testing for and diagnosis of latent TB increased despite a decrease in outbreak-associated active TB cases. Acceptance of latent TB treatment was >90%, which in our estimation was attributable to the universal support of the shelter providers. Regular inspections of facilities by public health staff provided frequent opportunities for ongoing education about TB and the need to maintain vigilance despite the downward trend in case numbers.
The primary challenge that remains is finding the substantial numbers of persons with untreated latent TB who are at risk for active TB but no longer in the shelter system. Because of this population, we anticipate that episodic cases of active TB with the outbreak genotype will occur. However, if the comprehensive guidelines now in place are properly implemented, reemergence of TB with the outbreak strain among shelter residents, as was seen in 2014, can be prevented. In addition, efforts are under way to increase the identification of persons with HIV (either not diagnosed or in persons not receiving care) and facilitate the rapid initiation of antiretroviral therapy along with the supportive measures required to maintain treatment adherence.
The presence of isoniazid resistance in the outbreak strain complicated treatment of active disease and latent infection (4,14). Unfortunately, 1 person with isoniazid-resistant TB diagnosed in 2008 later died of multidrug-resistant TB (15).
Homelessness presents several challenges to caring for persons with active TB and those who have been exposed to TB (https://www.cdc.gov/tb/topic/populations/homelessness/default.htm). Congregate living in shelters can provide an efficient means of transmission. Once tested, persons frequently move into, out of, and within shelter systems, making follow-up difficult. Also, shelter residents frequently lack a permanent place to store medications, which necessitates the use of directly observed therapy. In the outbreak we report, these challenges were overcome with a systematic operational approach focused on the entire population of persons experiencing homelessness, illustrating that such approaches can contribute to effective control of TB outbreaks within these populations.
Dr. Holland is an infectious disease physician with Emory University and also serves as chief clinical officer for Medical and Preventive Services at the Fulton County Board of Health. His research interests include cost-effectiveness analyses of public health interventions and implementation science.
References
- Powell KM, VanderEnde DS, Holland DP, Haddad MB, Yarn B, Yamin AS, et al. Outbreak of drug-resistant Mycobacterium tuberculosis among homeless people in Atlanta, Georgia, 2008–2015. Public Health Rep. 2017;132:231–40. DOIPubMedGoogle Scholar
- Leslie K. Reed wants to seize Peachtree-Pine by eminent domain. Atlanta Journal-Constitution. 2015 Aug 11 [cited 2019 Jan 18]. https://www.ajc.com/news/local-govt--politics/reed-wants-seize-peachtree-pine-eminent-domain
- Nandi PWM, Andrews T, Sales RM, McMichael J, Hampton KH, Goswami ND. Engaging homeless service providers in educational efforts during a tuberculosis outbreak in Atlanta. J Ga Public Health Assoc. 2016;6(Suppl 1):279–82 [cited 2018 Dec 28]. https://augusta.openrepository.com/augusta/bitstream/10675.2/621602/1/Nandi_2016_6S_2.pdf
- American Thoracic Society. Targeted tuberculin testing and treatment of latent tuberculosis infection. MMWR Recomm Rep. 2000;49(RR-6):1–51.PubMedGoogle Scholar
- Centers for Disease Control and Prevention. Reported tuberculosis in the United States, 2015 [cited 2018 Dec 20]. https://www.cdc.gov/tb/statistics/reports/2015/default.htm
- Centers for Disease Control and Prevention (CDC). Public health dispatch: tuberculosis outbreak in a homeless population—Portland, Maine, 2002-2003. MMWR Morb Mortal Wkly Rep. 2003;52:1184.PubMedGoogle Scholar
- Centers for Disease Control and Prevention (CDC). Tuberculosis transmission in a homeless shelter population—New York, 2000-2003. MMWR Morb Mortal Wkly Rep. 2005;54:149–52.PubMedGoogle Scholar
- Centers for Disease Control and Prevention (CDC). Tuberculosis outbreak associated with a homeless shelter - Kane County, Illinois, 2007-2011. MMWR Morb Mortal Wkly Rep. 2012;61:186–9.PubMedGoogle Scholar
- Centers for Disease Control and Prevention (CDC). Notes from the field: tuberculosis cluster associated with homelessness—Duval County, Florida, 2004-2012. MMWR Morb Mortal Wkly Rep. 2012;61:539–40.PubMedGoogle Scholar
- Centers for Disease Control and Prevention (CDC). Notes from the field: outbreak of tuberculosis associated with a newly identified Mycobacterium tuberculosis genotype—New York City, 2010-2013. MMWR Morb Mortal Wkly Rep. 2013;62:904.PubMedGoogle Scholar
- Curtis AB, Ridzon R, Novick LF, Driscoll J, Blair D, Oxtoby M, et al. Analysis of Mycobacterium tuberculosis transmission patterns in a homeless shelter outbreak. Int J Tuberc Lung Dis. 2000;4:308–13.PubMedGoogle Scholar
- Lathan M, Mukasa LN, Hooper N, Golub J, Baruch N, Mulcahy D, et al. Cross-jurisdictional transmission of Mycobacterium tuberculosis in Maryland and Washington, D C, 1996-2000, linked to the homeless. Emerg Infect Dis. 2002;8:1249–51. DOIPubMedGoogle Scholar
- National Tuberculosis Controllers Association; Centers for Disease Control and Prevention (CDC). Guidelines for the investigation of contacts of persons with infectious tuberculosis. Recommendations from the National Tuberculosis Controllers Association and CDC. MMWR Recomm Rep. 2005;54(RR-15):1–47.PubMedGoogle Scholar
- Blumberg HM, Burman WJ, Chaisson RE, Daley CL, Etkind SC, Friedman LN, et al.; American Thoracic Society, Centers for Disease Control and Prevention and the Infectious Diseases Society. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167:603–62. DOIPubMedGoogle Scholar
- Kalokhe AS, Shafiq M, Lee JC, Metchock B, Posey JE, Ray SM, et al. Discordance in Mycobacterium tuberculosis rifampin susceptibility. Emerg Infect Dis. 2012;18:537–9. DOIPubMedGoogle Scholar
Figures
Cite This ArticleOriginal Publication Date: February 13, 2019
Table of Contents – Volume 25, Number 3—March 2019
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
David P. Holland, Fulton County Board of Health, Medical and Preventive Services, 10 Park Place South SE, Atlanta, GA 30303, USA
Top