Volume 25, Number 7—July 2019
Dispatch
Nontuberculous Mycobacteria, Botswana, 2011–2014
Abstract
We documented a 6-fold increase in the frequency of nontuberculous mycobacteria isolated from clinical samples in Botswana during 2011–2014. Because antituberculosis treatment is often initiated only on the basis of acid-fast bacilli smear-positive microscopy results, some patients with nontuberculous mycobacterial infections might have received inappropriate treatment.
Nontuberculous mycobacteria (NTM) isolated from clinical samples are often classified as contaminants (1). However, some NTM species are pathogenic to humans (2) and, in some parts of the world, cause more illness than infection with Mycobacterium tuberculosis does (3).
In Botswana, as in many other developing countries, patients with acid-fast bacilli–positive sputum are presumed to be infected with M. tuberculosis and are treated with antituberculosis agents (4), even though acid-fast bacilli smear microscopy does not distinguish between M. tuberculosis and NTM, and most antituberculosis drugs might not be effective against NTM (5). This observation, along with the number of increasing reports of NTM worldwide (1,4,6–9), prompted this study.
During October 2015, we retrospectively analyzed 36,242 electronic records from 2011–2014 that were stored at the National Tuberculosis Reference Laboratory (Gaborone, Botswana) (Table). The records represented specimens referred for tuberculosis (TB) culture from 52 facilities across the country. Demographic parameters comprised age and sex. We compared proportions by calculating z values from observed frequencies, then used an online calculator to derive p values.
The proportions of M. tuberculosis and NTM cases during the study period were comparable (5.6% vs. 5.5%). This finding creates a high possibility of misdiagnosis and inappropriate treatment of M. tuberculosis/NTM cases. Most (74.5%) NTM samples were sputum; 22% were gastric aspirates, and the remaining 3.4% were obtained from other body sites. Of specimens from which NTM was isolated, 53.3% were from male patients and 43.4% were from female patients; for 3.1%, patient sex was not captured. The first specimen submitted per patient was analyzed. From the analysis we removed duplicate samples that might have resulted from submission of samples for follow-up from the same patient.
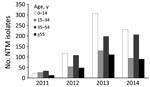
One third (33.5%) of samples from which NTM was isolated were from patients 0–14 years of age, followed by 27.3% from patients 35–54 years of age (Figure 1). For at least 207 (10.4%) of the 1,999 NTM samples, the age of the patient was not captured.
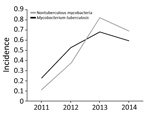
Overall, the proportion of isolated NTM increased 6-fold, from 0.113/100,000 population in 2011 to 0.693/100,000 in 2014 (the estimated Botswana population in 2017 was 2.292 million). Moreover, isolation of NTM significantly increased during 3 periods: 2011–2012, 2012–2013, and 2011–2014 (p<0.0001 for each period). We found no significant change in NTM occurrence for 2013–2014 (Figure 2).
Before 2013, NTM was not speciated in Botswana. In 2013, a total of 324 (39.4%) of 823 NTM isolates were speciated. Of these 324 samples, 161 (49.7%) were M. intracellulare, the NTM species most frequently isolated, followed by M. malmoense (17 [5.2%] of 324) and M. gordonae (15 [4.6%] of 324). Seventy-eight (24.0%) isolates could be identified only as Mycobacterium spp. because of a limitation of the assay used. Seventy-four (46.0%) patients in whom M. intracellulare was isolated were also HIV positive, suggesting that NTM might be an important opportunistic pathogen in HIV infection. M. avium-intracellulare has also been reported to cause most pulmonary infections in several studies (8,10,11). These reports are useful because failure to speciate NTM isolates or conduct drug susceptibility testing might be overlooking an emerging pathogen with potential to cause illness and even death in HIV-infected persons.
Most samples were from male patients, a finding consistent with findings by Moore et al. (1). This finding has been attributed to risk factors such as increased rates of smoking and chronic obstructive pulmonary disease among men (1). However, investigators in the United States observed a female predominance (3).
We observed a high occurrence of NTM in patients 35–54 years of age. We also noted an unexpected increase of >30% in reported NTM cases in children <14 years of age, which might represent a peculiar childhood vulnerability to NTM infections.
According to the Botswana National Tuberculosis Programme Manual, no information is available in Botswana about the prevalence of disseminated NTM (12). Our study provides basic but crucial data that may stimulate discussion toward policy change in the laboratory investigation and treatment of NTM isolates for better patient care.
The change in 2011 in the use of media from Lowenstein-Jensen to MGIT (Beckton-Dickinson, https://www.bd.com) culture might have contributed to an increase in NTM identification. A similar study by Chihota et al. (13) yielded more NTM after such a change. However, if the increase in this study resulted from a change in media, only the first year would have recorded an increase, followed by a stabilizing trend in subsequent years. Therefore, the increasing trend we observed reflects a convincing climb in NTM cases. Furthermore, our results suggest that patients with NTM might have received unnecessary TB treatment (7). This finding underscores the need to unequivocally identify mycobacterial isolates because NTM appears to be more frequently encountered (9).
Our study has limitations. Our results might not reflect the prevalence of NTM in the country because the samples analyzed comprised only persons suspected to have TB. This fact notwithstanding, the National Tuberculosis Referral Laboratory is the sole laboratory in Botswana that performs culture and thus represents a reasonable sampling of NTM countrywide among persons suspected to have TB infection. The prevalence of NTM might therefore be higher than that reported in this study.
We propose that mycobacterial isolates be routinely speciated and subjected to drug susceptibility testing. We base this proposal on our observation that NTM is isolated at nearly the same frequency as M. tuberculosis and is isolated predominantly in children <14 years of age. Treatment of NTM as M. tuberculosis underestimates the clinical significance of NTM and is likely to negatively affect treatment outcomes and inflate reports of M. tuberculosis.
Ms. Mbeha is a Laboratory Scientific Officer at the National Tuberculosis Reference Laboratory in Gaborone, Botswana. Her primary research interests include tuberculosis pathogenesis and treatment effectiveness.
Acknowledgment
We thank the Botswana Ministry of Health and Wellness for allowing access to the data used in this study.
References
- Moore JE, Kruijshaar ME, Ormerod LP, Drobniewski F, Abubakar I. Increasing reports of non-tuberculous mycobacteria in England, Wales and Northern Ireland, 1995-2006. BMC Public Health. 2010;10:612. DOIPubMedGoogle Scholar
- Northern Territory Department of Health. Guidelines for the control of nontuberculous mycobacteria in the Northern Territory [cited 2018 Dec 10]. https://hdl.handle.net/10137/527
- Cassidy PM, Hedberg K, Saulson A, McNelly E, Winthrop KL. Nontuberculous mycobacterial disease prevalence and risk factors: a changing epidemiology. Clin Infect Dis. 2009;49:e124–9. DOIPubMedGoogle Scholar
- Lai CC, Tan CK, Chou CH, Hsu HL, Liao CH, Huang YT, et al. Increasing incidence of nontuberculous mycobacteria, Taiwan, 2000-2008. Emerg Infect Dis. 2010;16:294–6. DOIPubMedGoogle Scholar
- Zhang ZY, Sun ZQ, Wang ZL, Hu HR, Wen ZL, Song YZ, et al. Identification and pathogenicity analysis of a novel non-tuberculous mycobacterium clinical isolate with nine-antibiotic resistance. Clin Microbiol Infect. 2013;19:91–6. DOIPubMedGoogle Scholar
- Kwon YS, Koh WJ. Diagnosis of pulmonary tuberculosis and nontuberculous mycobacterial lung disease in Korea. Tuberc Respir Dis (Seoul). 2014;77:1–5. DOIPubMedGoogle Scholar
- Wu J, Zhang Y, Li J, Lin S, Wang L, Jiang Y, et al. Increase in nontuberculous mycobacteria isolated in Shanghai, China: results from a population-based study. PLoS One. 2014;9:
e109736 . DOIPubMedGoogle Scholar - O’Brien RJ, Geiter LJ, Snider DE Jr. The epidemiology of nontuberculous mycobacterial diseases in the United States. Results from a national survey. Am Rev Respir Dis. 1987;135:1007–14.PubMedGoogle Scholar
- Novosad S, Henkle E, Winthrop KL. The challenge of pulmonary nontuberculous mycobacterial infection. Curr Pulmonol Rep. 2015;4:152–61. DOIPubMedGoogle Scholar
- Choudhri S, Manfreda J, Wolfe J, Parker S, Long R. Clinical significance of nontuberculous mycobacteria isolates in a Canadian tertiary care center. Clin Infect Dis. 1995;21:128–33. DOIPubMedGoogle Scholar
- Marras TK, Chedore P, Ying AM, Jamieson F. Isolation prevalence of pulmonary non-tuberculous mycobacteria in Ontario, 1997 2003. Thorax. 2007;62:661–6. DOIPubMedGoogle Scholar
- Botswana Ministry of Health. The National Tuberculosis Programme manual. 7th ed. Gaborone (Botswana): The Ministry; 2011.
- Chihota VN, Grant AD, Fielding K, Ndibongo B, van Zyl A, Muirhead D, et al. Liquid vs. solid culture for tuberculosis: performance and cost in a resource-constrained setting. Int J Tuberc Lung Dis. 2010;14:1024–31.PubMedGoogle Scholar
Figures
Table
Cite This ArticleOriginal Publication Date: May 15, 2019
Table of Contents – Volume 25, Number 7—July 2019
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Modisa Sekhamo Motswaledi, University of Botswana, School of Allied Health Professions, Block 246/214, Gaborone, Botswana
Top