Volume 26, Number 1—January 2020
Dispatch
Geographic Distribution and Incidence of Melioidosis, Panama1
Abstract
Melioidosis is an infection caused by Burkholderia pseudomallei. Most cases occur in Southeast Asia and northern Australia; <100 cases have been reported in the Americas. We conducted a retrospective study and identified 12 melioidosis cases in Panama during 2007–2017, suggesting possible endemicity and increased need for surveillance.
Burkholderia pseudomallei, a gram-negative bacillus found in the environment of some tropical and subtropical regions, is the etiologic agent of melioidosis (1–3). Most melioidosis cases in the world are reported from Southeast Asia and northern Australia; only sporadic cases are reported from other regions (4–6). In the Americas, <100 acquired cases were identified from 1947 through June 2015. Only 3 cases were reported from Panama, 1 each in 1947, 1948, and 2011. However, cases were reported in Antioquia, Colombia (1,7,8), near the border with Panama. Melioidosis might be misdiagnosed and underreported because of the lack of diagnostic resources in the rural areas where cases are most likely to occur (9,10).
People become infected with B. pseudomallei through inoculation in compromised derma, inhalation, or ingestion. Some evidence suggests ingestion is associated with bacteremia, even though ingestion is considered an uncommon pathway (2,3,11). Clinical manifestations of melioidosis are diverse and may include localized cutaneous infection, pneumonia, involvement of bones and joints, intraabdominal abscesses, sepsis, and even death (2,12). Diagnosis is usually made through blood cultures, but the bacterium often is misidentified as B. thailandensis or B. cepacia (10,13).
Current treatment for melioidosis includes an induction phase of 2–6 weeks with intravenous ceftazidime (or carbapenem for more severe cases), followed by a 2–6-month eradication phase using oral trimethoprim/sulfamethoxazole (TMP/SMX) or doxycycline. Doxycycline previously has been used for eradication, but recent studies suggest TMP/SMX is more effective (2,3).
During the previous 10 years, cases of melioidosis have been identified in different regions of Panama. The aim of this study is to describe the clinical signs and symptoms and geographic distribution of melioidosis in Panama to elucidate the current status of the disease in the Americas.
We conducted a retrospective review of medical records from 2007–2017 from 2 national tertiary level hospitals in Panama City. Hospital Santo Tomás and Complejo Hospitalario Metropolitano Dr. Arnulfo Arias Madrid (CHMDrAAM) are the 2 main referral hospitals for Panama and are in the capital city.
We reviewed specimen registries from the microbiology laboratories at each institution; we also identified 1 case from a poster presented at a national scientific meeting. We included patients who had a culture-positive report for B. pseudomallei and a clinical diagnosis of melioidosis at discharge. We excluded 2 patients with culture-positive results for B. pseudomallei because their clinical diagnoses were not related to their test results.
The microbiology laboratories of Hospital Santo Tomás and CHDrAAM identified B. pseudomallei strains from blood culture by using BacT/ALERT 3D Microbial Identification System (bioMérieux, https://www.biomerieux.com). Both laboratories also obtained isolates of B. pseudomallei from clinical specimens inoculated in Columbia agar prepared with 5% sheep blood and in MacConkey agar. Both laboratories used the VITEK 2 (bioMérieux) system to identify strains, which were then sent to the national reference laboratory at Instituto Conmemorativo Gorgas de Estudios de la Salud in Panama City, Panama, for microbiology confirmation and antimicrobial susceptibility testing.
We used a standardized form to collect data and then entered data into an Excel (Microsoft, https://www.microsoft.com) database for descriptive analysis. The Institutional Review Board of Hospital Santo Tomás reviewed and approved this study.
We identified 12 cases that occurred during 2007–2017: 8 in Hospital Santo Tomás and 4 in CHMDrAAM. We obtained medical records for all but 1 case, for which we obtained data from a poster presented at the 37th American College of Physicians Annual Central America Chapter Meeting in Panama City, Panama, in 2015 (14).
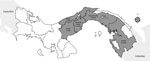
The mean age of cases was 50.3 years (SD ±12 years); most (9/12) patients were male. We noted bacteremia and sepsis in most (8/12) cases, pneumonia in 6 cases, and intraabdominal abscesses in 4 cases. Other signs and symptoms included endocarditis, meningitis, osteomyelitis, and septic arthritis (Table). Diabetes mellitus was the predominant risk factor. Most patients came from rural areas or suburbs of Panama City (Figure), and none reported travel outside of Panama.
All cases occurred during the rainy season, which is May–November in Panama. Five patients (41.7%) died while hospitalized; these patients had the most severe clinical manifestations of the disease, bacteremia, pneumonia, and septic shock, similar to cases reported from Central America (15).
Rapid microbiologic identification of B. pseudomallei is necessary to initiate appropriate, life-saving treatments. However, laboratory results can take >48 hours, delaying appropriate antimicrobial drug therapy. Of the 7 patients in this study who survived, records showed they were treated with TMP/SMX or doxycycline, but the length of antimicrobial drug treatments were not noted in the records.
The increase in reports of melioidosis in the Americas requires greater awareness of this disease among clinicians, especially those caring for patients with diabetes. Melioidosis often is misdiagnosed as pulmonary tuberculosis and scrofula (10); we found 2 misidentified clinical cases in our study. More studies are needed to identify specific high-risk areas and transmission routes in the Americas. Such insights can inform earlier clinical suspicion and guide the formulation of prevention strategies.
Because the clinical signs and symptoms of melioidosis are nonspecific, microbiologic identification is crucial to diagnosis. Thus, improved laboratory capacity is critical to improve patient outcomes in affected areas to aid epidemiologic and antibiotic susceptibility surveillance efforts. Collaboration among countries in the region could drive efforts to describe the origins of this disease and the actual prevalence in the Americas.
Our study has limitations because we collected data retrospectively and only included the most severe cases in Panama. Melioidosis occurs more frequently in rural areas, and cases might not be identified because of the lack of laboratory or diagnostic tools. We provide a perspective on the processes that hinder our knowledge of this disease in Panama, such as lack of surveillance data and inadequate laboratory capacity. Our data justify the need for increased surveillance for melioidosis and reinforce the need for complete epidemiologic data and adequate strain storage for further genetic analysis. Epidemiologic studies of seroprevalence, environmental sampling, and increased access to PCR techniques and broth microdilution testing are needed to determine whether B. pseudomallei is endemic to Panama and to improve treatment outcomes.
Dr. Araúz is an infectious disease specialist in Hospital Santo Tomás and professor of internal medicine at Universidad de Panama. Her primary research interest is in tropical diseases with emphases on melioidosis, HIV, histoplasmosis, and tuberculosis.
Acknowledgment
We thank the infectious diseases, pulmonology, intensive care, and internal medicine departments of Hospital Santo Tomás for their collaboration. We thank the microbiology laboratories of Hospital Santo Tomás and CHMDrAAM, and the National Public Health Reference Central Laboratory of Instituto Conmemorativo Gorgas de Estudios de la Salud for information related to the strains. We also thank Edgardo Brid for facilitating information related to his poster presentation on a case of melioidosis.
References
- Benoit TJ, Blaney DD, Doker TJ, Gee JE, Elrod MG, Rolim DB, et al. A review of melioidosis cases in the Americas. Am J Trop Med Hyg. 2015;93:1134–9. DOIPubMedGoogle Scholar
- Currie BJ. Melioidosis: evolving concepts in epidemiology, pathogenesis, and treatment. Semin Respir Crit Care Med. 2015;36:111–25. DOIPubMedGoogle Scholar
- Wiersinga WJ, Virk HS, Torres AG, Currie BJ, Peacock SJ, Dance DAB, et al. Melioidosis. Nat Rev Dis Primers. 2018;4:17107. DOIPubMedGoogle Scholar
- Perumal Samy R, Stiles BG, Sethi G, Lim LHK. Melioidosis: Clinical impact and public health threat in the tropics. PLoS Negl Trop Dis. 2017;11:
e0004738 . DOIPubMedGoogle Scholar - Chewapreecha C, Holden MT, Vehkala M, Välimäki N, Yang Z, Harris SR, et al. Global and regional dissemination and evolution of Burkholderia pseudomallei. Nat Microbiol. 2017;2:16263. DOIPubMedGoogle Scholar
- Currie BJ, Dance DA, Cheng AC. The global distribution of Burkholderia pseudomallei and melioidosis: an update. Trans R Soc Trop Med Hyg. 2008;102(Suppl 1):S1–4. DOIPubMedGoogle Scholar
- Montúfar FE, Ochoa JE, Ortega H, Franco L, Montúfar MC, Monsalve A, et al. “Melioidosis in Antioquia, Colombia: an emerging or endemic disease? A cases series”. Int J Infect Dis. 2015;37:50–7. DOIPubMedGoogle Scholar
- Adames E, Mendez A, Barrios J. Hepatic and skin abscess by Burkholderia pseudomallei [in Spanish]. Rev méd cient. 2013;26:34–44.
- Limmathurotsakul D, Golding N, Dance DAB, Messina JP, Pigott DM, Moyes CL, et al. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat Microbiol. 2016;1:15008. DOIGoogle Scholar
- Kingsley PV, Arunkumar G, Tipre M, Leader M, Sathiakumar N. Pitfalls and optimal approaches to diagnose melioidosis. Asian Pac J Trop Med. 2016;9:515–24. DOIPubMedGoogle Scholar
- Lim C, Peacock SJ, Limmathurotsakul D. Association between activities related to routes of infection and clinical manifestations of melioidosis. Clin Microbiol Infect. 2016;22:79.e1–3. DOIPubMedGoogle Scholar
- Currie BJ, Ward L, Cheng AC. The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year Darwin prospective study. PLoS Negl Trop Dis. 2010;4:
e900 . DOIPubMedGoogle Scholar - Lau SK, Sridhar S, Ho CC, Chow WN, Lee KC, Lam CW, et al. Laboratory diagnosis of melioidosis: past, present and future. Exp Biol Med (Maywood). 2015;240:742–51. DOIPubMedGoogle Scholar
- Brid E. Melioidosis: pancreatitis y endocarditis; presentación poco común. In: Abstracts of the XXXVII American College of Physicians Annual Central America Chapter Meeting; Panama; 2015 February 27-28.
- Sanchez-Villamil JI, Torres AG. Melioidosis in Mexico, Central America, and the Caribbean. Trop Med Infect Dis. 2018;3:24. DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleOriginal Publication Date: December 02, 2019
1Preliminary results from this study were presented at IDWeek, October 26–30, 2016, New Orleans, Louisiana, USA.
2These authors contributed equally to this article.
3Current affiliation: Hospital del Niño, Panama City, Panama.
Table of Contents – Volume 26, Number 1—January 2020
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Ana B. Araúz, Hospital Santo Tomás, Avenida Balboa y Calle Justo Arosemena, Panama City, Panama
Top