Volume 26, Number 11—November 2020
Research Letter
Burkholderia pseudomallei in Soil, US Virgin Islands, 2019
Cite This Article
Citation for Media
Abstract
The distribution of Burkholderia pseudomallei in the Caribbean is poorly understood. We isolated B. pseudomallei from US Virgin Islands soil. The soil isolate was genetically similar to other isolates from the Caribbean, suggesting that B. pseudomallei might have been introduced to the islands multiple times through severe weather events.
Burkholderia pseudomallei is a gram-negative soil-dwelling bacterium and the causative agent of melioidosis (1). B. pseudomallei is endemic to tropical regions around the world (1), but its environmental distribution in the Caribbean remains poorly understood. Although it is rare but ecologically established in Puerto Rico (2,3), it has not been isolated from the environment in the neighboring US Virgin Islands (USVI). After the 2017 Caribbean hurricane season, melioidosis developed in 3 persons in the USVI (4), 2 in St. Thomas and 1 in St. John. We aimed to determine whether, as this cluster suggests, B. pseudomallei might be endemic to the USVI.
We collected 480 soil and 100 freshwater samples from 29 sites (24 terrestrial and 5 freshwater) on the 3 main USVI islands (i.e., St. Thomas, St. John, and St. Croix) during January 20–April 17, 2019. We selected study sites to maximize geographic distribution across the islands and epidemiologic connection to melioidosis cases in humans (Appendix Figure 1). These efforts followed consensus guidelines for environmental sampling of B. pseudomallei (5) and methods previously reported (2) with 4 modifications: we collected 20 samples per site; we collected soil samples in 2 linear transects of 10 samples each; we collected 150 mL water per sample; and we used half of each sample for our analysis (the other half was archived). Although we strove for a sampling depth of 30 cm in soil, this was impossible at some sites because of rocks and debris (Appendix Table 1). We placed environmental samples in Ashdown’s liquid media for Burkholderia spp. enrichment (2). After enrichment, we extracted DNA using QiaAmp kits (QIAGEN, https://www.qiagen.com) and screened it using a B. pseudomallei–specific TaqMan assay (ThermoFisher Scientific, https://www.thermofisher.com) (6,7). We cultured samples to isolate pure B. pseudomallei and generate whole-genome sequences (WGSs). We conducted a phylogenetic analysis as previously described (2) and conducted genetic typing on these WGSs, WGSs from the 3 patients with melioidosis from USVI in 2017, and 43 additional B. pseudomallei WGSs available in GenBank from the Caribbean, the Americas, and Africa (Appendix Table 2).
We isolated B. pseudomallei from only 1 (»4%) of 24 soil sites, a prevalence resembling that of nearby Puerto Rico (2), where another study isolated B. pseudomallei from 2 soil samples collected at only 1 (2%) of 50 sampled sites. We obtained the B. pseudomallei–positive sample from site 122 (Appendix Figure 1), which was adjacent to a paved roadway 76 meters above sea level on eastern St. John. We collected the soil sample, which was composed of dry gravelly loam and had a pH of 6.9, from a depth of 30 cm (8) (Appendix; Appendix Table 1, Figure 2).
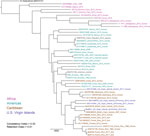
Our phylogenetic analysis assigned the 4 isolates (3 from patients, 1 from the environment) from the USVI to a monophyletic clade with all other B. pseudomallei isolates from the Caribbean (except 1 from Aruba) (Figure). However, none of the 4 isolates from the USVI were close genomic matches. These isolates differed by 6,355–10,115 single-nucleotide polymorphisms (SNPs) in the core genome, exhibiting more genomic diversity than B. pseudomallei isolates within Puerto Rico and Martinique (Figure). The 2019 soil and 2017 human isolates from St. John were not closely related (differing by 10,115 core genome SNPs), suggesting multiple introductions of B. pseudomallei to this island. The closest genomic match to the St. John soil isolate (differing by 170 core genome SNPs) was a 2007 isolate from Road Town, Tortola, British Virgin Islands (9). Although the dispersal mechanism of B. pseudomallei to this region is unknown, a dispersal event between these 2 locations (»11 km) might have been caused by aerosolization of B. pseudomallei during an extreme weather event, such as a hurricane (10). This mechanism of long-distance dispersal might also explain why the 2017 isolate from St. John is more closely related to isolates from Martinique than to the other isolates from USVI; this patient from the USVI was infected shortly after hurricane Maria (4). We placed the 2 isolates, despite differing by 6,355 core genome SNPs, from patients on St. Thomas in a single subclade; this pattern might suggest long-term endemicity on this island. However, these scenarios are based on an analysis of a relatively small number of B. pseudomallei WGSs from the Caribbean.
Our findings demonstrate that B. pseudomallei is rare in the environment in the USVI. The 2017 cases of melioidosis and the soil isolate from St. John indicate this bacterium might be ecologically established in the USVI. Additional environmental sampling will determine the environmental distribution of B. pseudomallei in the USVI, aiding the development of public health strategies to mitigate the risk for melioidosis.
Mr. Stone is a research project coordinator in the Pathogen and Microbiome Institute at Northern Arizona University. His research interests include molecular genetics and ecology of infectious diseases, population genetics of disease vectors, and public health.
Acknowledgments
We thank M. Martz, B. Schmidt, N. Bratsch, A. Jones, K. Guidry, K. Soria, A. Nunnally, and K. Sheridan for laboratory assistance. We also thank J. Jou, V. Burke-Frances, and L. deWilde for their help with field collections on St. Croix, M. Mayo for source data for the MSHR7398 and MSHR7400 isolates, and D. Mateos-Corral for additional location data for the 2007 melioidosis case in the British Virgin Islands.
Funding for this project was provided by the Centers for Disease Control and Prevention through award no. 75D30118C00607.
References
- Currie BJ. Melioidosis: evolving concepts in epidemiology, pathogenesis, and treatment. Semin Respir Crit Care Med. 2015;36:111–25. DOIPubMedGoogle Scholar
- Hall CM, Jaramillo S, Jimenez R, Stone NE, Centner H, Busch JD, et al. Burkholderia pseudomallei, the causative agent of melioidosis, is rare but ecologically established and widely dispersed in the environment in Puerto Rico. PLoS Negl Trop Dis. 2019;13:
e0007727 . DOIPubMedGoogle Scholar - Doker TJ, Sharp TM, Rivera-Garcia B, Perez-Padilla J, Benoit TJ, Ellis EM, et al. Contact investigation of melioidosis cases reveals regional endemicity in Puerto Rico. Clin Infect Dis. 2015;60:243–50. DOIPubMedGoogle Scholar
- Guendel I, Ekpo LL, Hinkle MK, Harrison CJ, Blaney DD, Gee JE, et al. Melioidosis after Hurricanes Irma and Maria, St. Thomas/St. John District, US Virgin Islands, October 2017. Emerg Infect Dis. 2019;25:1952–5. DOIPubMedGoogle Scholar
- Limmathurotsakul D, Dance DA, Wuthiekanun V, Kaestli M, Mayo M, Warner J, et al. Systematic review and consensus guidelines for environmental sampling of Burkholderia pseudomallei. PLoS Negl Trop Dis. 2013;7:
e2105 . DOIPubMedGoogle Scholar - Wiersinga WJ, Virk HS, Torres AG, Currie BJ, Peacock SJ, Dance DAB, et al. Melioidosis. Nat Rev Dis Primers. 2018;4:17107. DOIPubMedGoogle Scholar
- Novak RT, Glass MB, Gee JE, Gal D, Mayo MJ, Currie BJ, et al. Development and evaluation of a real-time PCR assay targeting the type III secretion system of Burkholderia pseudomallei. J Clin Microbiol. 2006;44:85–90. DOIPubMedGoogle Scholar
- US Department of Agriculture. Web Soil Survey. 2018 [cited 2019 Sept 16]. https://websoilsurvey.sc.egov.usda.gov
- Corral DM, Coates AL, Yau YC, Tellier R, Glass M, Jones SM, et al. Burkholderia pseudomallei infection in a cystic fibrosis patient from the Caribbean: a case report. Can Respir J. 2008;15:237–9. DOIPubMedGoogle Scholar
- Cheng AC, Jacups SP, Gal D, Mayo M, Currie BJ. Extreme weather events and environmental contamination are associated with case-clusters of melioidosis in the Northern Territory of Australia. Int J Epidemiol. 2006;35:323–9. DOIPubMedGoogle Scholar
Figure
Cite This ArticleOriginal Publication Date: October 08, 2020
Table of Contents – Volume 26, Number 11—November 2020
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
David M. Wagner, Northern Arizona University, PO Box 4073, Flagstaff, AZ 86011, USA
Top