Volume 27, Number 10—October 2021
Research Letter
SARS-CoV-2 Neutralization Resistance Mutations in Patient with HIV/AIDS, California, USA
Abstract
We report persistent severe acute respiratory syndrome coronavirus 2 infection in a patient with HIV/AIDS; the virus developed spike N terminal domain and receptor binding domain neutralization resistance mutations. Our findings suggest that immunocompromised patients can harbor emerging variants of severe acute respiratory syndrome coronavirus 2.
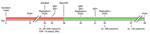
In December 2020, a 61-year-old woman living with HIV/AIDS was tested for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection at a community testing center in California, USA; she produced an anterior nasal swab sample that tested positive by reverse transcription PCR (RT-PCR). At the time of sample collection, she had a 10-day history of nonproductive cough, and was not receiving antiretroviral therapy (Figure). Her CD4 count was 13 cells/μL and HIV-1 viral load was 262,000 copies/mL. She never required hospitalization for SARS-CoV-2 infection. Thirty days after symptom onset, she no longer had respiratory symptoms and underwent SARS-CoV-2 screening upon admission to Stanford Hospital (Stanford, CA, USA) for treatment of a severe decubitus ulcer. Her nasopharyngeal swab (NPS) sample tested positive for SARS-CoV-2 by RT-PCR (cycle threshold [Ct] value 17.6). We detected minus-strand viral RNA, indicating active viral replication (1). On day 33, three days after admission to Stanford Hospital, antibody testing showed plasma positive for IgM against SARS-CoV-2 spike receptor binding domain (RBD) but negative for IgG against SARS-CoV-2 spike S1 (2,3). She began antiretroviral therapy (ART) 38 days after symptom onset. On day 45, an NPS sample tested positive (Ct 16.6) for SARS-CoV-2 with detectable minus-strand RNA.
Because the patient had ongoing viral replication, we conducted whole-genome sequencing using archived nucleic acids from the NPS samples collected on days 30 and 45. We enriched the viral genome using laboratory-developed multiplex RT-PCR reactions that generated multiple overlapping amplicons of ≈1,200 bp. We prepared fragment libraries using NEBNext DNA Library Prep reagents (New England Biolabs, https://www.neb.com) according to the manufacturer’s instructions; we sequenced the libraries on Illumina MiSeq with single-end 150-cycle sequencing using MiSeq Reagent Kit v3 (https://www.illumina.com). We assembled the consensus sequences and identified mutations using a custom bioinformatics pipeline and SARS-CoV-2 isolate Wuhan-Hu-1 (GenBank accession no. NC_45512.2) as reference. For these 2 samples we observed mean whole-genome coverage of 963× (day 30) and 894× (day 45). We used the consensus sequences from the day 30 (GISAID accession no. EPI_ISL_2009056) and day 45 (GISAID accession no. EPI_ISL_2009057) samples to query the GISAID CoVserver (https://www.gisaid.org) and Phylogenetic Assignment of Named Global Outbreak LINeages (PANGOLIN, https://pangolin.cog-uk.io) to determine clade and lineage.
The sequence from the sample taken on day 30 revealed a G clade, B.1.234 lineage virus (Table). Because the day 45 sequence shares 18 mutations (8 synonymous and 10 nonsynonynmous) with the day 30 sequence and is the most closely related sequence to the day 30 sequence in GISAID, we believe the day 45 sequence probably evolved from the day 30 sequence. The day 45 sequence contained additional spike mutations, including C15F (variant percent 44.3%), del141_144 (17.5%), Y248N (13.4%), ins248_Y/LLSFN (44.5%), and E484Q (67.7%) (Table). The cysteine residue at position 15 (C15) in the spike N terminal domain (NTD) is linked by a disulfide bond to C136; mutations at either of these positions alter this bond and reduce neutralization by monoclonal antibodies (4). Deletions and insertions in the NTD are also involved in immune escape, including the common del141_144 mutation and insertions at position Y248 (5). The E484Q mutation is located in the RBD domain of the spike gene and is also found in the Kappa variant of interest (i.e., B.1.617.1) (6). Viruses harboring E484Q have reduced susceptibility to monoclonal antibodies, convalescent plasma, and vaccinee plasma (7,8).
The patient showed SARS-CoV-2 IgG seroconversion on day 51, thirteen days after initiating ART. SARS-CoV-2 antibody isotypes typically follow a similar time-course; IgM, IgA, and IgG usually become detectable ≈14 days after illness onset (2). This patient’s delayed IgG class switch was probably caused by HIV/AIDS-associated B-cell dysfunction; we hypothesize that the ineffective IgM response might have selected for the observed spike mutations (9). On day 55, seventeen days after initiating ART, the patient’s HIV-1 viral load was 330 copies/mL. An NPS sample collected that day was negative for minus-strand SARS-CoV-2 RNA, and the viral load had decreased >1,000-fold (Ct 27.2). The patient’s SARS-CoV-2 infection remained asymptomatic throughout her hospitalization. On day 93, she produced an NPS sample that tested negative for SARS-CoV-2 RNA.
In summary, we describe an HIV-positive patient who had a prolonged course of asymptomatic, active SARS-CoV-2 infection leading to the emergence of NTD and RBD mutations associated with reduced antibody neutralization. Our findings add to the accumulating evidence that immunocompromised persons, including persons living with HIV/AIDS, might host ongoing SARS-CoV-2 replication that could enable the development of variants of concern/interest (F. Karim, unpub. data, https://www.medrxiv.org/content/10.1101/2021.06.03.21258228v1). The emergence of multiple spike mutations in this patient over a relatively short timeframe (i.e., 15 days) further highlights the potential role of persons living with uncontrolled HIV as possible sources of SARS-CoV-2 variants. Finally, these findings emphasize the need to diagnose HIV in the >6 million infected persons worldwide who are unaware of their status and provide them with accessible ART. These interventions are critical for overall global health and might also contribute to controlling the COVID-19 pandemic.
Dr. Hoffman is an infectious diseases fellow at the Stanford University School of Medicine, Stanford, California, USA. His research interests include global health equity and clinical research to benefit underserved populations.
Acknowledgment
We thank the healthcare providers and laboratory staff at Stanford Health Care for their high-quality work and dedication to patient care.
References
- Hogan CA, Huang C, Sahoo MK, Wang H, Jiang B, Sibai M, et al. Strand-specific reverse transcription PCR for detection of replicating SARS-CoV-2. Emerg Infect Dis. 2021;27:632–5. DOIPubMedGoogle Scholar
- Röltgen K, Powell AE, Wirz OF, Stevens BA, Hogan CA, Najeeb J, et al. Defining the features and duration of antibody responses to SARS-CoV-2 infection associated with disease severity and outcome. Sci Immunol. 2020;5:
eabe0240 . DOIPubMedGoogle Scholar - Wang H, Wiredja D, Yang L, Bulterys PL, Costales C, Röltgen K, et al. Case-control study of individuals with discrepant nucleocapsid and spike protein SARS-CoV-2 IgG results. Clin Chem. 2021;67:977–86. DOIPubMedGoogle Scholar
- McCallum M, De Marco A, Lempp FA, Tortorici MA, Pinto D, Walls AC, et al. N-terminal domain antigenic mapping reveals a site of vulnerability for SARS-CoV-2. Cell. 2021;184:2332–2347.e16. DOIPubMedGoogle Scholar
- Harvey WT, Carabelli AM, Jackson B, Gupta RK, Thomson EC, Harrison EM, et al.; COVID-19 Genomics UK (COG-UK) Consortium. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol. 2021;19:409–24. DOIPubMedGoogle Scholar
- Verghese M, Jiang B, Iwai N, Mar M, Sahoo MK, Yamamoto F, et al. A SARS-CoV-2 variant with L452R and E484Q neutralization resistance mutations. J Clin Microbiol. 2021;59:
e0074121 . DOIPubMedGoogle Scholar - Edara VV, Pinsky BA, Suthar MS, Lai L, Davis-Gardner ME, Floyd K, et al. Infection and vaccine-induced neutralizing-antibody responses to the SARS-CoV-2 B.1.617 variants. N Engl J Med. 2021;
NEJMc2107799 . DOIPubMedGoogle Scholar - Greaney AJ, Loes AN, Crawford KHD, Starr TN, Malone KD, Chu HY, et al. Comprehensive mapping of mutations in the SARS-CoV-2 receptor-binding domain that affect recognition by polyclonal human plasma antibodies. Cell Host Microbe. 2021;29:463–476.e6. DOIPubMedGoogle Scholar
- Moir S, Fauci AS. B cells in HIV infection and disease. Nat Rev Immunol. 2009;9:235–45. DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleOriginal Publication Date: July 23, 2021
Table of Contents – Volume 27, Number 10—October 2021
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Benjamin A. Pinsky, Stanford University School of Medicine, 3375 Hillview Ave, Rm 2913, Palo Alto, CA 94304, USA
Top