Volume 28, Number 8—August 2022
Dispatch
Culling of Urban Norway Rats and Carriage of Bartonella spp. Bacteria, Vancouver, British Columbia, Canada
Cite This Article
Citation for Media
Abstract
We investigated the effects of culling on Bartonella spp. bacteria carriage among urban rats in Canada. We found that the odds of Bartonella spp. carriage increased across city blocks except those in which culling occurred. Removing rats may have prevented an increase in Bartonella spp. prevalence, potentially lowering human health risks.
Urban Norway rats (Rattus norvegicus) carry Bartonella spp., which are bacteria transmitted among rats and to humans through vectors including fleas (1). Infection in humans can result in fever, fatigue, myalgia, and endocarditis (2). In Vancouver, British Columbia, Canada, a serosurvey of residents of an underresourced neighborhood found that 3% of participants had been exposed to B. tribocorum (3), a species found in rats in this neighborhood (4), suggesting that rats may be an exposure source for humans in this area.
Although aimed at decreasing disease risks, culling methods (i.e., lethal removal) may increase zoonotic pathogen prevalence by altering normal behaviors that modify pathogen transmission (5,6). We sought to determine whether culling rats altered Bartonella spp. prevalence in rats and their fleas in the Downtown Eastside neighborhood of Vancouver. The University of British Columbia’s Animal Care Committee (A14-0265) approved study procedures.
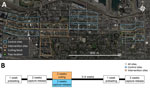
We trapped rats in 12 study sites (5 intervention, 7 control), each comprising 3 contiguous city blocks (36 total blocks) (Figure, panel A) during June 2016–January 2017 (Appendix). We placed 10 live traps (Tomahawk Live Traps, https://www.livetrap.com) in the alley of each block. We conducted the experiment in 3 trapping phases: before, during, and after the intervention (Figure, panel B). Before and after the intervention, we captured rats, gave each a numbered ear tag, and released it to its capture site. In the center block of intervention sites culling occurred during the second trapping phase. In flanking blocks (those adjacent to the intervention block) and control blocks, no culling occurred (Figure, panel A).
We collected blood from all rats via jugular puncture under isoflurane anesthesia. We collected fleas by brushing the coat.
We identified fleas to species (7), and pooled <5 fleas per rat. We extracted DNA from rat blood and fleas using the DNEasy Blood and Tissue Kit (QIAGEN, https://www.qiagen.com). We tested DNA extracts for Bartonella spp. by real-time PCR. For rat blood, we used primers to detect a 380-bp segment of the citrate synthase gene (gltA) (8). For fleas, we used a probe-based real-time PCR assay to detect a 302-bp fragment of the ssrA gene (9). We conducted our analysis as described in Himsworth et al. (10).
We used generalized linear mixed models to assess the relationship between the intervention and Bartonella spp. carriage. We controlled for spatial clustering by city block as a random effect. We assessed positive or negative carriage by rats (model A) and fleas (model B) and the number of fleas per rat (model C). We analyzed carriage models A and B by logistic regression and model C by negative binomial regression. For all models, the intervention variable consisted of 4 categories indicating when rats or fleas were caught: before the intervention in all blocks; after the intervention in control blocks; after the intervention in flanking blocks; and after the intervention in intervention blocks.
We used a hypothesis-testing model building approach to estimate the effect of the intervention while accounting for covariates (Table). We retained covariates if they confounded the relationship between the intervention and the outcome (i.e., if they changed the effect of any level of the intervention by >10% or if their association with the outcome and intervention had p<0.25). We also kept independent predictors of the outcome if they significantly improved the model, as indicated by a likelihood ratio test result of p<0.05; that test compared 2 nested models, each with the intervention variable and all confounders present, but with and without the potential predictor variable.
We trapped 512 Norway rats; 206 (40.2%) of them had fleas. The median number of fleas per rat was 0 (range 0–58; mean 1.18). All fleas were Nosopsyllus fasciatus. We obtained blood from 454 rats; 90 (20%) tested positive for Bartonella spp. We tested 201 flea pools; 86 (42.8%) tested positive for Bartonella spp. (Table). In the final model A, which contained the variables season, presence of Bartonella spp.–positive fleas, and wound presence as covariates, the odds of Bartonella spp. carriage were significantly higher among rats caught after the intervention in control blocks (odds ratio [OR] 2.68; 95% CI 1.22–6.67) and flanking blocks (OR 7.26; 95% CI 1.56–38.17), but not in the intervention blocks (OR 2.03; 95% CI 0.22–15.41), when compared with the odds of carriage before the intervention in all block types (Table). We saw no association between the intervention and the number of fleas per rat or Bartonella spp. carriage by fleas.
The prevalence of Bartonella spp. bacteria among rats in this neighborhood has been shown to increase in the fall (4). Our study suggests that culling rats may have prevented this increase within the blocks where culling occurred.
Removing rats may change how individual rats interact within colonies, which alters pathogen transmission. Bartonella spp. transmission via fleas (1) requires close contact among individual rats. Rats burrow communally, establishing a network of chambers with some shared nests (11). Those nests promote close contact among rats and act as a source of fleas that spend time in the nest (12). Decreased rat population density may lessen nest sharing and behaviors such as social grooming, thereby reducing opportunities for fleas to transmit Bartonella spp. among individual rats. A reduction in Bartonella spp. prevalence may decrease exposure risk for humans, but the relationship between rodents, vectors, pathogens, and humans is complex (13). For example, although a previous study revealed that residents in this neighborhood had been exposed to Bartonella spp. (3), it is unclear whether this exposure was associated with rats and to what extent humans encounter fleas. Furthermore, for other fleaborne pathogens such as Yersinia pestis (agent of the plague), culling rats may increase disease transmission to humans as fleas seek new hosts (14). Understanding how rat abundance and rat removal impacts intraspecies and interspecies dynamics and pathogen prevalence is necessary to anticipate management impacts on pathogen transmission.
Whereas our intervention involved removing rats and their fleas, we did not observe a change in the number of fleas on rats. The steady number suggests that culling did not reduce flea abundance, perhaps because N. fasciatus fleas also reside in the burrows, such that the number of fleas per rat does not reflect the total number of fleas in a city block (12). It is possible that our intervention removed a negligible proportion of the flea population. In addition, we did not observe a change in the odds of Bartonella spp. carriage among fleas. A past study in this neighborhood showed that Bartonella spp. carriage among rats was not related to flea presence or abundance; therefore, the role of N. fasciatus fleas in the ecology of Bartonella spp. in this ecosystem remains enigmatic (15).
Our findings counter a study of Leptospira interrogans using the same experimental design, in which culling was associated with an increased odds of infection among rats (5). This difference is likely attributable to differences in transmission; L. interrogans is spread via urine (13) and Bartonella spp. via fleas (1). Culling may alter a variety of social interactions (e.g., fighting, nest-sharing, grooming) which affect the spread of these pathogens differently. Together, these studies illustrate the complexity of managing rat-associated zoonoses; the intervention may have opposite effects on different pathogens. Indeed, past literature has shown that culling wildlife to control zoonoses can have unpredictable consequences (6) and that ecosystem-based approaches that manage the human–wildlife interface may be more effective.
Dr. Byers is the deputy director of the British Columbia Node of the Canadian Wildlife Health Cooperative and a university research associate at Simon Fraser University. At time this research was conducted, she was a PhD student at the University of British Columbia. Her research focus is using systems approaches to derive actionable solutions to One Health issues affecting wildlife, people, and the environment.
Acknowledgments
We thank the Vancouver Area Network of Drug Users for their assistance in enacting this study. We also thank Charles Krebs, Bobby Corrigan, and Michael Whitlock for their suggestions regarding the design of this study. Finally, we thank Geoffrey Knaub, Sophia Kontou, and Emilia Mackowiak for their assistance in the field.
This study was funded in part by the Public Scholars Initiative at the University of British Columbia (K.A.B.), a Natural Sciences and Engineering Research Council of Canada Discovery Grant (D.M.P.), and the Public Health Agency of Canada (J.E.H.).
References
- Gutiérrez R, Krasnov B, Morick D, Gottlieb Y, Khokhlova IS, Harrus S. Bartonella infection in rodents and their flea ectoparasites: an overview. Vector Borne Zoonotic Dis. 2015;15:27–39. DOIPubMedGoogle Scholar
- Kosoy M, Bai Y, Sheff K, Morway C, Baggett H, Maloney SA, et al. Identification of Bartonella infections in febrile human patients from Thailand and their potential animal reservoirs. Am J Trop Med Hyg. 2010;82:1140–5. DOIPubMedGoogle Scholar
- McVea DA, Himsworth CG, Patrick DM, Lindsay LR, Kosoy M, Kerr T. Exposure to rats and rat-associated Leptospira and Bartonella species among people who use drugs in an impoverished, inner-city neighborhood of Vancouver, Canada. Vector Borne Zoonotic Dis. 2018;18:82–8. DOIPubMedGoogle Scholar
- Himsworth CG, Bai Y, Kosoy MY, Wood H, DiBernardo A, Lindsay R, et al. An investigation of Bartonella spp., Rickettsia typhi, and Seoul hantavirus in rats (Rattus spp.) from an inner-city neighborhood of Vancouver, Canada: is pathogen presence a reflection of global and local rat population structure? Vector Borne Zoonotic Dis. 2015;15:21–6. DOIPubMedGoogle Scholar
- Lee MJ, Byers KA, Donovan CM, Bidulka JJ, Stephen C, Patrick DM, et al. Effects of culling on Leptospira interrogans carriage by rats. Emerg Infect Dis. 2018;24:356–60. DOIPubMedGoogle Scholar
- Donnelly CA, Woodroffe R, Cox DR, Bourne FJ, Cheeseman CL, Clifton-Hadley RS, et al. Positive and negative effects of widespread badger culling on tuberculosis in cattle. Nature. 2006;439:843–6. DOIPubMedGoogle Scholar
- Holland GP. The fleas of Canada, Alaska, and Greenland (Siphonaptera). Mem Entomol Soc Can. 1985;117(S130):3–632. DOIGoogle Scholar
- Hornok S, Beck R, Farkas R, Grima A, Otranto D, Kontschán J, et al. High mitochondrial sequence divergence in synanthropic flea species (Insecta: Siphonaptera) from Europe and the Mediterranean. Parasit Vectors. 2018;11:221. DOIPubMedGoogle Scholar
- Diaz MH, Bai Y, Malania L, Winchell JM, Kosoy MY. Development of a novel genus-specific real-time PCR assay for detection and differentiation of Bartonella species and genotypes. J Clin Microbiol. 2012;50:1645–9. DOIPubMedGoogle Scholar
- Himsworth CG, Byers KA, Fernando C, Speerin L, Lee MJ, Hill JE. When the sum of the parts tells you more than the whole: The advantage of using metagenomics to characterize Bartonella spp. infections in Norway rats (Rattus norvegicus) and their fleas. Front Vet Sci. 2020;7:
584724 . DOIPubMedGoogle Scholar - Calhoun JB. The ecology and sociology of the Norway rat. Bethesda, MD: US Department of Health, Education, and Welfare, Public Health Service; 1963.
- Bitam I, Dittmar K, Parola P, Whiting MF, Raoult D. Fleas and flea-borne diseases. Int J Infect Dis. 2010;14:e667–76. DOIPubMedGoogle Scholar
- Meerburg BG, Singleton GR, Kijlstra A. Rodent-borne diseases and their risks for public health. Crit Rev Microbiol. 2009;35:221–70. DOIPubMedGoogle Scholar
- Keeling MJ, Gilligan CA. Metapopulation dynamics of bubonic plague. Nature. 2000;407:903–6. DOIPubMedGoogle Scholar
- Himsworth CG, Byers KA, Whelan T, Bai Y, Kosoy MY. Flea presence and abundance are not predictors of Bartonella tribocorum carriage in Norway rats (Rattus norvegicus) from an underserved neighborhood of Vancouver, Canada. Vector Borne Zoonotic Dis. 2021;21:121–4. DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleOriginal Publication Date: July 08, 2022
1These first authors contributed equally to this article.
Table of Contents – Volume 28, Number 8—August 2022
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Kaylee Byers, School of Population and Public Health, University of British Columbia, 2206 East Mall, Vancouver, BC V6T 1Z3, Canada
Top