Volume 29, Number 10—October 2023
Dispatch
Imported Toxigenic Corynebacterium Diphtheriae in Refugees with Polymicrobial Skin Infections, Germany, 2022
Abstract
During August–December 2022, toxigenic Corynebacterium diphtheriae was isolated from 25 refugees with skin infections and 2 refugees with asymptomatic throat colonization at a refugee reception center in Germany. None had systemic toxin-mediated illness. Of erosive/ulcerative skin infections, 96% were polymicrobial. Erosive/ulcerative wounds in refugees should undergo testing to rule out cutaneous diphtheria.
Diphtheria is a potentially lethal upper respiratory tract infection that causes systemic illness associated with toxemia. Cases are mostly caused by toxigenic Corynebacterium diphtheriae and, rarely, by C. ulcerans through animal-to-human transmission (1). Although <500 cases have been detected in Europe during 2010–2019 (2), outbreaks have been reported in resource-limited settings (e.g., in refugee camps or in settings of waning immunization coverage) (3,4). During June–October 2022, a total of 371 diphtheria cases were detected in Europe; most (147 cases) were in Germany. Ongoing cases in 2023 and a fatal case in Belgium in June 2023 reported by the European Centre for Disease Prevention and Control (ECDC) highlight the need for further awareness (https://www.ecdc.europa.eu/en/publications-data/communicable-disease-threats-report-2-8-july-2023-week-27; https://www.ecdc.europa.eu/en/publications-data/communicable-disease-threats-report-30-january-5-february-2023-week-5; https://www.ecdc.europa.eu/en/publications-data/communicable-disease-threats-report-11-17-june-2023-week-24).
Since 2015, the University Medical Centre Freiburg has run an outpatient clinic at the refugee reception center in Freiburg, which in late 2022 detected an unusually high number of skin infections in refugees. After the initial case of cutaneous C. diphtheriae infection was detected, the University Medical Centre consulted with the local health authorities, and subsequent patients with skin wounds or erosive/ulcerative lesions were tested for throat colonization and skin infection with C. diphtheriae. Contacts of patients with confirmed cases (roommates, other close contacts) were identified and screened for C. diphtheriae skin infection and throat colonization. Our retrospective analysis was approved by the ethics committee of the University Medical Centre Freiburg (22-1493-S1-retro). Anonymized photographs were taken with the verbal consent of the patients.
In the beginning of the analysis period, we detected C. diphtheriae by using Columbia blood agar with fosfomycin plates and, after sufficient production, with tellurite agar. We confirmed isolates as C. diphtheriae by using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and using Bruker MALDI Biotyper (5). We detected the diphtheria toxin gene by using a conventional PCR assay (6). We sent all isolates for confirmation to the consiliary laboratory at Landesamt für Gesundheit Bayern (LGL Bayern, Oberschleißheim, Germany), where Elek test and multilocus-sequence typing (MLST) were additionally performed for several isolates.
During August 1, 2022, through December 31, 2022, C. diphtheriae was detected in 27 refugees. Of those, 25 had sought care because of nonhealing skin lesions or wounds. Screening of 154 contacts (throat swabs) identified 2 asymptomatic carriers of C. diphtheriae without skin lesions. All patients were male, and most were young (mean 24 years, range 18–49 years). Three patients stated that they were minors but were classified as adults by local health authorities. Most patients were born in Afghanistan (15 persons) or Syria (11 persons); 1 refugee reported Morocco as his country of birth. Of the 18 (67%) refugees who were asked about their escape route, all stated that they had fled via Balkan states (Table 1; Appendix Table 1).
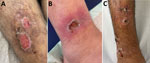
Cutaneous lesions persisted for a mean of 22.5 days; ≈70% persisted >14 days, and the overall range was wide, 3–60 days. Available detailed histories of the cause and circumstances of the primary manifestation indicated waterway crossings and forest habitation as the probable infection mode. The lesions were predominantly localized at the extremities: lower thigh (17 [63%]), feet and ankles (11 [41%]), hands (8 [32%]), and upper thigh (2 [7%]). In addition, genital lesions were observed in 2 refugees (7%) (Table 1; Appendix Table 1). Skin wounds appeared as partly punched-out, partly erosive lesions with erythematous margins (Figure panels A, B). Grayish mucous membranes or purulent lesions were detectable in isolated cases only.
Clinical differentiation between skin infections caused by Staphylococcus aureus, Streptococcus pyogenes, or both (Figure panel C) or wounds with evidence of C. diphtheriae was difficult. In addition to C. diphtheriae, both S. aureus and S. pyogenes were detected in 21 (84%) of the 25 refugees. In 3 (12%) refugees, C. diphtheriae and S. pyogenes were detected; in 1, only C. diphtheriae was detected. All 25 skin infections were colonized with toxin-producing C. diphtheriae as shown by positive PCR for the tox gene or positive Elek test through the consiliary laboratory (Table 2; Appendix Table 1). The 2 cases of C. diphtheriae throat colonization were nontoxigenic. Among the 21 S. aureus isolates, 10 (48%) were methicillin resistant. Of the 24 S. pyogenes isolates, 3 (13%) showed resistance to clindamycin (Table 2). According to current European Committee on Antimicrobial Susceptibility Testing (https://www.eucast.org) recommendations, all C. diphtheriae isolates showed in vitro susceptibility to penicillin at increased exposure, and all but 2 isolates showed sensitivity to erythromycin or clindamycin (7). MLST was available for 16 isolates; sequence types 377 or 574 were identified 6 times, and sequence type 384 was identified 4 times. We established no correlation between country of origin and sequence type.
Detection of toxin-producing C. diphtheriae from throat swab samples was successful in 5 (20%) of the 25 patients with cutaneous lesions. No systemic illnesses associated with toxemia were observed in our cohort.
Our report adds details about the clinical picture of cutaneous diphtheria among refugees from Afghanistan, Syria, and Morocco in Germany. In this cohort, 100% of cutaneous infections were caused by toxigenic C. diphtheriae, which is a higher proportion than the 27% toxigenic cutaneous infections reported in 270 cases published over the past 65 years (Appendix Table 2). This magnitude indicates a common source of infection or increased risks for transmission while fleeing (8,9).
Nearly all skin infections in this cohort were polymicrobial, caused by C. diphtheriae, S. aureus, and S. pyogenes. Co-infections with S. pyogenes have been reported in the literature for 151 (56%) cases and with S. aureus for 110 (41%); methicillin resistance was noted in 19 (7%) cases (Appendix Table 2). Only 1 report indicates a rate of co-pathogens in the magnitude of that found in our cohort (10). Similar to a previous report from Germany, the isolates were broadly drug susceptible (11); susceptibility to penicillin seemed to be higher than that reported from Spain during 2014–2019 (12).
Another finding was that one fifth of the refugees with cutaneous diphtheria were concurrently colonized with toxigenic C. diphtheriae in the throat while remaining systemically asymptomatic. Such concurrent throat plus skin colonization may be highly relevant for transmission and should be identified.
Although in their rapid risk assessment the ECDC considered the overall risk for the residing population in refugee-accepting countries to be very low, several steps are crucial for preventing spread and casualties (13). Toxin-mediated systemic disease can be effectively prevented by universal immunization against diphtheria toxin. Refugee infants are particularly at risk because of the reported low rates of receiving a third dose of diphtheria-tetanus-pertussis vaccine in Afghanistan (81%) and Syria (48%) (14). Furthermore, waning immunity against vaccine-preventable diseases, especially for pertussis and diphtheria, is reported where antibody levels drop to prevaccination levels 5–6 years after vaccination (15).
Our report, in conjunction with material from ECDC, could be informative for migrants and healthcare workers with regard to identifying cutaneous diphtheria (13). These findings supplement recommendations for contact tracing, screening for throat colonization, and using personal protective equipment when changing dressings or taking swab specimens.
Workers at refugee reception centers should pay attention to chronic erosive/ulcerative wounds in refugees. They should conduct adequate microbiological investigations to rule out cutaneous diphtheria, even if S. aureus or S. pyogenes have already been identified, and should screen persons, including contact persons, for throat colonization. Booster vaccinations or full immunizations against diphtheria toxin and antimicrobial prophylaxis should be given in accordance with ECDC guidelines in risk settings when cases of diphtheria are suspected (13).
Dr. Spielberger works in pediatric infectious diseases. He has a strong interest in improving care for refugee minors and is head of the pediatric outpatient clinic at the refugee reception center in Freiburg.
Acknowledgment
We thank the local health authorities in Freiburg, Germany, and the consiliary laboratory for diphtheria at LGL Bayern, Oberschleißheim, Germany, for discussing transmission and infection control issues.
References
- Sharma NC, Efstratiou A, Mokrousov I, Mutreja A, Das B, Ramamurthy T. Diphtheria. Nat Rev Dis Primers. 2019;5:81. DOIPubMedGoogle Scholar
- Muscat M, Gebrie B, Efstratiou A, Datta SS, Daniels D. Diphtheria in the WHO European Region, 2010 to 2019. Euro Surveill. 2022;27:
2100058 . DOIPubMedGoogle Scholar - Hardy IR, Dittmann S, Sutter RW. Current situation and control strategies for resurgence of diphtheria in newly independent states of the former Soviet Union. Lancet. 1996;347:1739–44. DOIPubMedGoogle Scholar
- Polonsky JA, Ivey M, Mazhar MKA, Rahman Z, le Polain de Waroux O, Karo B, et al. Epidemiological, clinical, and public health response characteristics of a large outbreak of diphtheria among the Rohingya population in Cox’s Bazar, Bangladesh, 2017 to 2019: A retrospective study. PLoS Med. 2021;18:
e1003587 . DOIPubMedGoogle Scholar - Hsieh SY, Tseng CL, Lee YS, Kuo AJ, Sun CF, Lin YH, et al. Highly efficient classification and identification of human pathogenic bacteria by MALDI-TOF MS. Mol Cell Proteomics. 2008;7:448–56. DOIPubMedGoogle Scholar
- Nakao H, Popovic T. Development of a direct PCR assay for detection of the diphtheria toxin gene. J Clin Microbiol. 1997;35:1651–5. DOIPubMedGoogle Scholar
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters, version 13.1, 2023 [cited 2023 July 10]. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_13.1_Breakpoint_Tables.pdf
- Sing A, Heesemann J. Imported cutaneous diphtheria, Germany, 1997-2003. Emerg Infect Dis. 2005;11:343–4. DOIPubMedGoogle Scholar
- Kofler J, Ramette A, Iseli P, Stauber L, Fichtner J, Droz S, et al. Ongoing toxin-positive diphtheria outbreaks in a federal asylum centre in Switzerland, analysis July to September 2022. Euro Surveill. 2022;27:
2200811 . DOIPubMedGoogle Scholar - May MLA, McDougall RJ, Robson JM. Corynebacterium diphtheriae and the returned tropical traveler. J Travel Med. 2014;21:39–44. DOIPubMedGoogle Scholar
- Marosevic DV, Berger A, Kahlmeter G, Payer SK, Hörmansdorfer S, Sing A. Antimicrobial susceptibility of Corynebacterium diphtheriae and Corynebacterium ulcerans in Germany 2011-17. J Antimicrob Chemother. 2020;75:2885–93. DOIPubMedGoogle Scholar
- Hoefer A, Pampaka D, Herrera-León S, Peiró S, Varona S, López-Perea N, et al. Molecular and epidemiological characterization of toxigenic and nontoxigenic Corynebacterium diphtheriae, Corynebacterium belfantii, Corynebacterium rouxii, and Corynebacterium ulcerans isolates identified in Spain from 2014 to 2019. J Clin Microbiol. 2021;59:e02410–20. DOIPubMedGoogle Scholar
- European Center for Disease Prevention and Control. Increase of reported diphtheria cases among migrants in Europe due to Corynebacterium diphtheriae [cited 2023 Jul 10]. https://www.ecdc.europa.eu/en/publications-data/increase-reported-diphtheria-cases-among-migrants-europe-due-corynebacterium
- World Health Organization. WHO and UNICEF estimates of immunization coverage: 2021 revision [cited 2023 Feb 17]. https://www.who.int/teams/immunization-vaccines-and-biologicals/immunization-analysis-and-insights/global-monitoring/immunization-coverage/who-unicef-estimates-of-national-immunization-coverage
- Gao H, Lau EHY, Cowling BJ. Waning immunity after receipt of pertussis, diphtheria, tetanus, and polio-related vaccines: a systematic review and meta-analysis. J Infect Dis. 2022;225:557–66. DOIPubMedGoogle Scholar
Figure
Tables
Cite This ArticleOriginal Publication Date: August 24, 2023
1These senior authors contributed equally to this article.
Table of Contents – Volume 29, Number 10—October 2023
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Benedikt Daniel Spielberger, University Medical Center Freiburg, Mathildenstr. 1, 79106 Freiburg, Germany
Top