Volume 29, Number 5—May 2023
Dispatch
Severe Streptococcus equi Subspecies zooepidemicus Outbreak from Unpasteurized Dairy Product Consumption, Italy
Abstract
During November 2021–May 2022, we identified 37 clinical cases of Streptococcus equi subspecies zooepidemicus infections in central Italy. Epidemiologic investigations and whole-genome sequencing showed unpasteurized fresh dairy products were the outbreak source. Early diagnosis by using sequencing technology prevented the spread of life-threatening S. equi subsp. zooepidemicus infections.
Streptococcus equi subspecies zooepidemicus is a β-hemolytic streptococcus expressing Lancefield group C antigen and is 1 of 3 S. equi subspecies. S. equi subsp. zooepidemicus is an opportunistic pathogen that can infect domestic animals, pets, and wildlife (1–6). Sporadic human cases have been reported (7), characterized by clinical manifestations that vary from meningitis to sepsis. Human infection generally occurs through direct contact with infected animals or by consumption of contaminated unpasteurized milk or other dairy products (8–10). We report a large S. equi subsp. zooepidemicus outbreak in Italy.
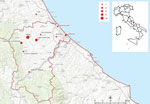
During November 2021–February 2022, S. equi subsp. zooepidemicus infections were detected in 18 hospitalized patients who resided in a limited area within the province of Pescara, Italy (Figure 1). A wide range of clinical manifestations were observed in the patients, including septicemia, pharyngitis, arthritis, uveitis, and endocarditis. Five of those patients died from severe meningitis. For most cases, S. equi subsp. zooepidemicus bacteremia was accompanied by >1 additional localized bacterial site. S. equi subsp. zooepidemicus was detected in various patient specimens, including articular effusions and cerebrospinal fluid, that were tested by hospital laboratories. A task force consisting of physicians, veterinarians, epidemiologists, scientists, microbiologists, and communication experts was created and coordinated by the local competent authority to evaluate the outbreak.
We conducted an active investigation of S. equi subsp. zooepidemicus cases, defined as patients with laboratory-confirmed infection. We identified 37 outbreak cases over a 7-month period. We completed interviews of 36 patients or their relatives by using telephone-administered questionnaires. Patients were 6–98 (median 79) years of age. Male (n = 16) and female (n = 21) patients were similarly represented (Table 1). We obtained 21 bacteria isolates from 19 hospitalized patients that were sent to Istituto Zooprofilattico Sperimentale in Teramo, Italy. We identified bacteria species by using a Biotyper matrix-assisted laser desorption/ionization time-of-flight mass spectrometer (Bruker, https://www.bruker.com).
We performed whole-genome sequencing for 21 isolates by using the Illumina platform (Illumina, https://www.illumina.com) with a setting of 300 cycles (150-bp paired-end reads) (11). After read quality checks, we confirmed all clinical strains were S. equi subsp. zooepidemicus by using the KmerFinder tool (12). We assigned sequence type (ST) 61 to trains by using a multilocus sequence typing scheme (13) (https://pubmlst.org/streptococcus-zooepidemicus).
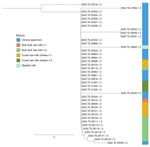
We confirmed correlations between human strains by performing single-nucleotide polymorphism (SNP) analysis through the US Food and Drug Administration Center for Food Safety and Applied Nutrition pipeline (14) and by using MinION technology (15) to obtain a hybrid genome from an outbreak strain as a reference. We found that all 21 clinical strains were closely related (0–3 SNPs), implying involvement of a unique source strain in the observed human cases. Moreover, the antimicrobial susceptibility test performed on all human strains gave identical results, confirming the hypothesis that 1 source strain was responsible for all human cases.
An extensive investigation was performed by public health, veterinary, and food hygiene services to identify the infection source. Epidemiologic analysis showed that 31 patients consumed soft or semi-soft cheeses purchased from local producers or dealers. A total of 8 local dairy food business operators were inspected. Samples of raw bulk milk (from cows and sheep), fresh cheese from unpasteurized and pasteurized milk, and water were obtained from each operator and sent to Istituto Zooprofilattico Sperimentale, Teramo, for bacteria detection. We cultured the samples and isolated Streptococcus spp. from sheep blood agar after incubation for 24 ±1 h at 37°C ±1°C in a 5% CO2 enriched atmosphere. We tested all samples by using Genesig real-time PCR kits (Primerdesign Ltd, http://www. primerdesign.co.uk). We performed species identification and whole-genome sequencing as described for human samples. We detected S. equi subsp. zooepidemicus in an unpasteurized bulk cow milk sample taken from 1 dairy producer selected within the outbreak area. That operator was then officially inspected and sampled by local competent authorities; a total of 18 S. equi subsp. zooepidemicus strains were isolated from 2 bulk milk tanks and 2 cured raw milk cheese samples. Genome sequences from those samples were also ST61; SNP analysis showed the 18 strains clustered with the clinical strains, indicating a strong correlation between the operator- and human-derived strains.
After S. equi subsp. zooepidemicus was identified as the pathogen responsible for the human cases, we reviewed data from the Istituto Zooprofilattico Sperimentale strain library. We found a strain that was isolated from the milk of a cow with mastitis in November 2021; the animal belonged to the same operator whose products tested positive. After sequencing, the strain was also identified as ST61 (Table 2) and clustered with the other trains isolated from human patients and raw milk products (Figure 2).
On the basis of epidemiologic and laboratory analyses, local competent authorities established measures to limit and prevent S. equi subsp. zooepidemicus spread by the end of February 2022. All dairy products were recalled from the market and local dealers and ripening cheeses were destroyed; local authorities required pasteurization of milk intended for cheese production.
We report epidemiologic, microbiologic, and genomic findings from a S. equi subsp. zooepidemicus outbreak that involved 37 patients in Italy. We found strong genomic correlation between strains isolated from case-patients, unpasteurized milk and dairy products, and milk from infected cows that clearly indicated a zoonotic infection source. Questionnaire and laboratory data showed that human infections were caused by consuming unpasteurized fresh cheese produced from infected milk cows. We were unable to trace the origin of infection back to specific dairy food business operator livestock. The farmer who had the positive cow referred to construction work in the barn during October–November 2021, which might have caused stress, predisposing the animal to mastitis. A possible reactivation of a latent S. equi subsp. zooepidemicus infection cannot be excluded. Identifying the source of infection in a relatively short time enabled a rapid response that prevented further cases in the community. However, the outbreak in Italy was considered the largest and most severe outbreak associated with the consumption of unpasteurized fresh cheese that has been reported.
In summary, our study implicates S. equi subsp. zooepidemicus as a possible zoonotic pathogen and highlights the bacterium’s virulence in humans. Awareness of individual anamnestic information and, in particular, possible contacts with domestic animals or recent consumption of unpasteurized dairy products was crucial for managing this outbreak. Equally important and of extreme value was the One Health interdisciplinary approach used to find solutions and solve community concerns. Further research is needed to gain insights into pathogenic characteristics of the strain responsible for the outbreak. Early diagnosis and identification of bacteria by using molecular methodologies will improve medical treatment outcomes, enable timely epidemiologic disease surveillance, and prevent spread of life-threatening infections.
Ms. Bosica is a biologist at the Istituto Zooprofilattico Sperimentale dell'Abruzzo e del Molise "G. Caporale" in Teramo, Italy. Her interests focus on food matrix analysis for major foodborne pathogens and genetically modified organisms.
References
- Skive B, Rohde M, Molinari G, Braunstein TH, Bojesen AM. Streptococcus equi subsp. zooepidemicus Invades and Survives in Epithelial Cells. Front Cell Infect Microbiol. 2017;7:465. DOIPubMedGoogle Scholar
- Costa MO, Lage B. Streptococcus equi subspecies zooepidemicus and sudden deaths in swine, Canada. Emerg Infect Dis. 2020;26:2522–4. DOIPubMedGoogle Scholar
- Sharp MW, Prince MJ, Gibbens J. S zooepidemicus infection and bovine mastitis. Vet Rec. 1995;137:128. DOIPubMedGoogle Scholar
- Las Heras A, Vela AI, Fernández E, Legaz E, Domínguez L, Fernández-Garayzábal JF. Unusual outbreak of clinical mastitis in dairy sheep caused by Streptococcus equi subsp. zooepidemicus. J Clin Microbiol. 2002;40:1106–8. DOIPubMedGoogle Scholar
- Pisoni G, Zadoks RN, Vimercati C, Locatelli C, Zanoni MG, Moroni P. Epidemiological investigation of Streptococcus equi subspecies zooepidemicus involved in clinical mastitis in dairy goats. J Dairy Sci. 2009;92:943–51. DOIPubMedGoogle Scholar
- Blum S, Elad D, Zukin N, Lysnyansky I, Weisblith L, Perl S, et al. Outbreak of Streptococcus equi subsp. zooepidemicus infections in cats. Vet Microbiol. 2010;144:236–9. DOIPubMedGoogle Scholar
- Baracco GJ. Infections caused by group C and G streptococcus (Streptococcus dysgalactiae subsp. equisimilis and others): epidemiological and clinical aspects. Microbiol Spectr. 2019;7:
7.2.32 . DOIPubMedGoogle Scholar - Kuusi M, Lahti E, Virolainen A, Hatakka M, Vuento R, Rantala L, et al. An outbreak of Streptococcus equi subspecies zooepidemicus associated with consumption of fresh goat cheese. BMC Infect Dis. 2006;6:36. DOIPubMedGoogle Scholar
- Francis AJ, Nimmo GR, Efstratiou A, Galanis V, Nuttall N. Investigation of milk-borne Streptococcus zooepidemicus infection associated with glomerulonephritis in Australia. J Infect. 1993;27:317–23. DOIPubMedGoogle Scholar
- Torres RSLA, Santos TZ, Bernardes AFL, Soares PA, Soares ACC, Dias RS. Outbreak of glomerulonephritis caused by Streptococcus zooepidemicus SzPHV5 type in Monte Santo de Minas, Minas Gerais, Brazil. J Clin Microbiol. 2018;56:e00845–18. DOIPubMedGoogle Scholar
- Chiaverini A, Guidi F, Torresi M, Acciari VA, Centorotola G, Cornacchia A, et al. Phylogenetic analysis and genome-wide association study applied to an Italian Listeria monocytogenes outbreak. Front Microbiol. 2021;12:
750065 . DOIPubMedGoogle Scholar - Larsen MV, Cosentino S, Lukjancenko O, Saputra D, Rasmussen S, Hasman H, et al. Benchmarking of methods for genomic taxonomy. J Clin Microbiol. 2014;52:1529–39. DOIPubMedGoogle Scholar
- Webb K, Jolley KA, Mitchell Z, Robinson C, Newton JR, Maiden MCJ, et al. Development of an unambiguous and discriminatory multilocus sequence typing scheme for the Streptococcus zooepidemicus group. Microbiology (Reading). 2008;154:3016–24 . DOIPubMedGoogle Scholar
- Davis S, Pettengill JB, Luo Y, Payne J, Shpuntoff A, Rand H, et al. CFSAN SNP pipeline: an automated method for constructing SNP matrices from next-generation sequence data. PeerJ Comput Sci. 2015;1:
e20 . DOIGoogle Scholar - Neal-McKinney JM, Liu KC, Lock CM, Wu WH, Hu J. Comparison of MiSeq, MinION, and hybrid genome sequencing for analysis of Campylobacter jejuni. Sci Rep. 2021;11:5676. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: April 12, 2023
Table of Contents – Volume 29, Number 5—May 2023
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Alexandra Chiaverini, Istituto Zooprofilattico Sperimentale Abruzzo e Molise “G. Caporale,” Via Campo Boario, 64100, Teramo, Italy
Top