Volume 30, Number 5—May 2024
Research
Epidemiologic Survey of Crimean-Congo Hemorrhagic Fever Virus in Suids, Spain
Abstract
We conducted a cross-sectional study in wild boar and extensively managed Iberian pig populations in a hotspot area of Crimean-Congo hemorrhagic fever virus (CCHFV) in Spain. We tested for antibodies against CCHFV by using 2 ELISAs in parallel. We assessed the presence of CCHFV RNA by means of reverse transcription quantitative PCR protocol, which detects all genotypes. A total of 113 (21.8%) of 518 suids sampled showed antibodies against CCHFV by ELISA. By species, 106 (39.7%) of 267 wild boars and 7 (2.8%) of 251 Iberian pigs analyzed were seropositive. Of the 231 Iberian pigs and 231 wild boars analyzed, none tested positive for CCHFV RNA. These findings indicate high CCHFV exposure in wild boar populations in endemic areas and confirm the susceptibility of extensively reared pigs to CCHFV, even though they may only play a limited role in the enzootic cycle.
Crimean-Congo hemorrhagic fever virus (CCHFV; genus Orthonairovirus family Nairoviridae) is an emerging pathogen mainly transmitted by ticks of the genus Hyalomma (1,2). This arbovirus is the causative agent of Crimean-Congo hemorrhagic fever (CCHF), a severe and lethal zoonotic and hemorrhagic disease in humans (2,3). In Europe, human cases of CCHF have been traditionally reported only in southeastern countries (2,4). However, shortly after the virus was detected in Spain (western Europe) in Hyalomma lusitanicum ticks collected on red deer (Cervus elaphus) in 2010 (5), human CCHF clinical cases have been confirmed in western and southwestern Spain since 2013 (6–8).
Since 2010, endemic circulation of CCHFV has been reported in the Iberian Peninsula in H. lusitanicum ticks (9,10) and red deer, which are the primary host of adult specimens of this tick species (11). In this region, red deer populations usually share habitat and natural resources with other susceptible wild ungulate species, such as wild boar (Sus scrofa), another important natural host of adult H. lusitanicum ticks. This connection, together with the drastic increase of the wild boar population since 1990 in the Iberian Peninsula (12), may contribute to the spread and maintenance of the virus in Mediterranean ecosystems.
Few studies have assessed CCHFV circulation in red deer and wild boar (11,13–16). In addition, those 2 wild ungulate species usually cohabit with extensively managed domestic Iberian pigs; direct and indirect contact between those sympatric species are frequent (17). Iberian pig is a breed of the domestic pig autochthonous to the Iberian Peninsula; they are usually reared under extensive management conditions and fattened during the Montanera period (October‒February) by feeding on acorns within large, fenced areas. Most of the Iberian pig farms (80%) are in southwestern Spain, where circulation of CCHFV is high. The European Food Safety Authority (EFSA) has prioritized surveillance of CCHFV in pigs (18); however, data on exposure to CCHFV in pigs are lacking (19,20).The aim of our study was to analyze the circulation of CCHFV in wild boar and sympatric Iberian domestic pigs in southwestern Spain, a hotspot area of CCHFV, where CCHFV-positive ticks are present (5,21,22) and high seroprevalence has been reported in animal hosts (11,14).
Study Area and Sampling
In this cross-sectional study, we assumed a seroprevalence of 40.6% (11) for wild boar and calculated the sample size as 251; we assumed a seroprevalence of 50% (which provides the highest sample size in studies in which seroprevalence is unknown) for a sample of 267 pigs. We calculated those estimates with 95% CI and a desired precision of +6%. Whenever possible, we sampled 14 Iberian pigs in each farm to detect exposure with a 95% probability, assuming a minimum within-farm seroprevalence of 20%.
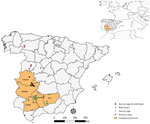
We sampled wild boar in 35 hunting states between the hunting seasons 2015‒16 and 2020‒21 in 5 provinces of southwestern Spain. In addition, we collected blood samples from Iberian pigs from 18 farms managed under extensive production systems during 2017‒and 2019 in the same study area (Figure). We collected blood samples from pigs at the slaughterhouse and from wild boar by puncture of the cavernous sinus of the dura mater (23). We obtained serum samples by blood centrifugation at 400 × g for 10 minutes and froze them until serologic and molecular analysis. We recorded epidemiologic information, including sex, age, origin, and year of sampling, from sampled animals whenever possible. We estimated age of wild boar on the basis of tooth replacement patterns. We obtained all Iberian pig samples at the end of the Montanera period.
Serologic Methods
We tested for antibodies against CCHFV using 2 different ELISAs, both based on recombinant CCHFV nucleoprotein (N) as described elsewhere (24). In brief, the first test was the CCHF Double Antigen Multispecies ELISA (IDvet Screen, https://www.innovative-diagnostics.com), which is based on the CCHFV IbAr10200 strain N of clade III (GenBank accession no. U88410). This commercial test was validated by analyzing several animal species, including pigs (25). The second ELISA was an in-house indirect ELISA based on the CCHFV Kosovo Hoti strain N belonging to clade V (GenBank accession no. DQ133507). For this study, we considered samples seropositive if CCHFV antibodies were detected by both ELISAs (double-reactive).
Molecular Detection
We analyzed a total of 462 serum samples, 231 available from each species, for molecular detection. We extracted RNA from serum samples using the NucleoMag Vet Kit (Macherey-Nagel, https://www.mn-net.com). We assessed the presence of CCHFV RNA by means of QuantiTect Probe RT-PCR Kit (QIAGEN, https://www.qiagen.com) using the multiplex quantitative RT-PCR described elsewhere (26), which can amplify a fragment of the short (S) segment of the 6 known CCHFV genotypes.
Statistical Analysis
We calculated seroprevalence and prevalence of active infection by dividing the number of seropositive animals by ELISA and positive animals by quantitative PCR by the total number of animals tested, using 2-sided exact binomial of 95% CI. We initially assessed associations between serologic results and explanatory variables by species using bivariate analysis with the Pearson χ2 or Fisher exact tests, as appropriate. We selected variables with p values <0.10 as potential risk factors. We evaluated collinearity between variables using the Cramer V coefficient, selecting the variable with the highest biologic plausibility if we obtained a correlation coefficient between variables >0.6 and p<0.05. Finally, we evaluated the influence of the selected explanatory variables on CCHFV seropositivity using a generalized linear mixed model (GLMM), assuming a binomial data distribution and the variable municipality as random effect. We considered the Akaike information criterion score for each model to select the most accurate. Variables with p<0.05 were statistically significant. We performed all statistical analyses using RStudio (https://github.com/rstudio/rstudio).
Of the 518 suids sampled, 71 animals tested positive exclusively through the commercial ELISA, whereas 7 tested positive solely through the in-house assay (24). Of note, a total of 113 of the 518 suids sampled showed antibodies against CCHFV by both ELISAs. Thus, the seroprevalence obtained in the preset study was 21.8% (95% CI 18.4%–25.5%; 113/518). By species, 106 (39.7%, 95% CI 34.0%–45.7%) of the 267 wild boar and 7 (2.8%, 95% CI 0.8%–4.8%) of the 251 Iberian pigs analyzed were seropositive (Table 1). We found significantly higher seroprevalence in wild boar than in pigs (relative risk 14.23, 95% CI 6.7–30.0; p<0.001).
We found antibodies against CCHFV in wild boar in 26 (70.3%) of the 37 hunting estates sampled and in the 5 provinces analyzed; seroprevalence ranged from 21.9% in Badajoz to 51.0% in Córdoba (Figure). We detected >1 seropositive wild boar in each of the hunting seasons (Table 1). We detected seropositivity in 2 (11.1%) of the 18 pig farms analyzed; within-farm seroprevalence was 5%‒70%. We identified seropositive pigs in 2 provinces sampled; seroprevalence ranged from 0.8% (95% CI 0–2.3%) in Córdoba to 7.8% (95% CI 1.8%–13.8%) in Cáceres (Table 1). We sampled 6/7 seropositive pigs in the province of Cáceres, in the same extensively managed pig farm.
The GLMM model identified age and sex as risk factors potentially associated with human exposure to CCHFV in wild boar (Table 2). We found significantly higher seropositivity in adults (49.0%; odds ratio (OR) 3.9, 95% CI 1.6–9.5) than in yearlings (20.0%). Female (46.0%) wild boar showed 2 times (OR 1.89, 95% CI 1.0–3.4) higher risk of exposure to the virus than males. We identified no risk factor in Iberian pigs by GLMM model. None of the 231 Iberian pigs (0.0%, 95% CI 0.0–1.6%) and 231 wild boars (0.0%, 95% CI 0.0–1.6%) analyzed tested positive to CCHFV RNA presence in their serum.
Despite extensive data on potential CCHFV hosts and their influence in the maintenance and transmission of this virus (27), information on the role of suids in the epidemiology of CCHFV worldwide is very limited. As of February 2024, all surveys conducted worldwide in wild boar have been done in Spain, except for 1 in Turkey, where 2.5% of wild boar were exposed to the virus (28). The seroprevalence detected in this species in our study (39.7%) is in accordance with the 40.6% obtained in this species in Doñana National Park in southwestern Spain (13) and indicates a high exposure of wild boar populations to CCHFV in this region of Spain. In contrast, lower frequencies of seropositivity were found in northeastern Spain, where 3.2% of wild boar showed antibodies against CCHFV (15), and eastern Spain, where 15.3% of wild boar had antibodies (16). Those spatial differences found in Spain are consistent with the risk gradient of exposure to CCHFV observed in wild boar on the Iberian Peninsula (14) and could be associated with certain climatic factors that can condition the density and abundance of the competent vector species throughout Spain. The presence of Hyalomma spp. ticks in northeastern Spain had not been described until recently (29,30) and in the eastern part of the country was detailed through a study conducted on primary care on bites from these ticks (31). In contrast, the presence of Hyalomma spp. ticks, including H. marginatum and H. lusitanicum, has been widely described in southwestern Spain (9,10,32,33).
This study provides evidence on the potential role of domestic pigs in the enzootic cycle of CCHFV. As of February 2024, the 2 serosurveys of pigs did not detect antibodies against CCHFV in any of the 25 sampled pigs from India or the 46 sampled in Egypt (19, 20). The seroprevalence obtained (2.8%) confirmed the susceptibility of Iberian pigs to CCHFV infection but indicate a low exposure of extensively raised populations to the virus in a hotspot area, thus suggesting a limited role of this species in the enzootic cycle of CCHFV on the Iberian Peninsula. Nonetheless, it is significantly different from the seroprevalence determined in wild boar. Of note, the period that Iberian pigs are extensively managed (October‒February) does not coincide with the period of main activity of Hyalomma spp. ticks (April‒June) (34). The pigs are exposed to the vectors for a few months, in contrast to wild boars’ exposure throughout their lives, which could explain the differences in seroprevalence between these species. In addition, this finding could be related to potential differences in susceptibility to viral infection between pigs and wild boar. Future experimental studies can clarify this hypothesis.
In the case of wild boar, we found that age and sex were associated with CCHFV exposure (Table 2). Adults were at 3.6 times higher risk of CCHFV exposure than yearlings, which could be associated with the higher probability of tick bites throughout the life of adult wild boar and with the persistence of CCHFV antibodies over time. This finding is in line with those of Cuadrado-Matías et al. (13) and with previous studies that linked the age of cattle with greater seropositivity to CCHFV antibodies (35–37). On the other hand, female wild boar had significantly higher seroprevalence than males. Previous studies found a higher proportion of female than male wild boar infested by ticks, possibly caused by behavioral differences (37). Similarly, other studies have found a higher proportion of CCHFV-seropositive female cattle (38,39). However, statistically significant differences in CCHFV seropositivity between sexes had not previously been found in the limited serosurveys carried out in wild boar. Additional studies in boar species are needed to evaluate the role of age and sex in CCHFV exposure.
We detected seropositivity every year and in all provinces sampled, indicating an endemic and widespread circulation of CCHFV in southwestern Spain. Of note, yearling animals were seropositive in all of the hunting seasons analyzed except 2020–2021. Although maternal antibodies in yearling mammals cannot be ruled out, our findings denote active circulation during the study period. On the other hand, wild boar from Córdoba province had the highest seroprevalence value; 51% of animals tested seropositive to CCHFV, which was higher than in Badajoz province where human cases have been reported (Figure). In Córdoba, the presence of CCHFV has already been demonstrated by the detection of CCHFV RNA in ticks (21,22), which would indicate that this region has a high circulation of the virus. Those data highlight the need to carry out studies throughout Spain to establish spatial distribution of CCHFV to promote surveillance and control programs in areas identified as hotspots. On the other hand, 6/7 (85.7%) seropositive pigs were sampled in 2018 in the same extensively managed pig farm in Cáceres. That finding indicates a nonhomogeneous spatiotemporal distribution pattern of the virus in this domestic species, which implies a possible role of the Iberian pig as accidental host rather than true host of CCHFV.
Finally, even though the quantitative PCR used in this study has the capacity to detect all the clades of the virus (26), we did not find CCHFV RNA in any of the 462 serum specimens tested. This result is consistent with those previously reported in other mammal species (40–42) and could be explained by the transient short period of viremia described (<7 days) in small mammals, small ruminants, and hares (43,44). However, we know of no studies evaluating viremia in suids, which would be necessary to establish their role as natural host of CCHFV.
In conclusion, the results obtained in this study suggest an endemic and widespread circulation of the virus in southwestern Spain. Specifically, these findings indicate high CCHFV exposure in wild boar populations in endemic areas. We report CCHFV exposure in domestic pigs, confirming the susceptibility of this species to the virus, although the low seroprevalence found indicates a limited role in the enzootic cycle of CCHFV in the Iberian and Mediterranean ecosystems. Therefore, we recommend the use of biosecurity measures for high-risk activities with this species to limit exposure to this pathogen. This study highlights the need to develop surveillance programs in suids to evaluate spatiotemporal changes in the circulation of CCHFV to prevent infection in humans.
Dr. Frías is a postdoctoral researcher at Animal Health and Zoonosis Research Group (GISAZ) at the University of Cordoba and the Clinical Virology and Zoonoses Group at the Maimonides Biomedical Research Institute of Cordoba. His main field of study is zoonotic emerging diseases.
Acknowledgments
We thank Sarah Knapp for excellent technical assistance.
This work has been partially supported by TED2021-132599B-C22 project, funded by MCIN/AEI/10.13039/501100011033 and by the European Union NextGenerationEU/Plan de Recuperación, Transformación y Resiliencia and by CIBER -Consorcio Centro de Investigación Biomédica en Red- (CB 2021), Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación and Unión Europea-NextGenerationEU. S.C.-S. was supported by a Formación de Profesorado Universitario grant from the Spanish Ministry of Universities (FPU19/06026). J.C.-G. is supported by the CIBER Consorcio Centro de Investigación Biomédica en Red- (CB21/13/00083), Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación and Unión Europea-NextGenerationEU. S.J.-R. is supported by a Juan de la Cierva contract (JDC2022-048850-I) funded by the Ministerio de Ciencia e Innovación/AEI/10.13039/501100011033 and the European Union NextGenerationEU/PRTR.
References
- Bartolini B, Gruber CE, Koopmans M, Avšič T, Bino S, Christova I, et al. Laboratory management of Crimean-Congo haemorrhagic fever virus infections: perspectives from two European networks. Euro Surveill. 2019;24:
1800093 . DOIPubMedGoogle Scholar - Messina JP, Pigott DM, Golding N, Duda KA, Brownstein JS, Weiss DJ, et al. The global distribution of Crimean-Congo hemorrhagic fever. Trans R Soc Trop Med Hyg. 2015;109:503–13. DOIPubMedGoogle Scholar
- Hawman DW, Feldmann H. Recent advances in understanding Crimean-Congo hemorrhagic fever virus. F1000Res. 2018;7:F1000 Faculty Rev-1715.
- Papa A, Weber F, Hewson R, Weidmann M, Koksal I, Korukluoglu G, et al. Meeting report: First International Conference on Crimean-Congo hemorrhagic fever. Antiviral Res. 2015;120:57–65. DOIPubMedGoogle Scholar
- Estrada-Peña A, Palomar AM, Santibáñez P, Sánchez N, Habela MA, Portillo A, et al. Crimean-Congo hemorrhagic fever virus in ticks, Southwestern Europe, 2010. Emerg Infect Dis. 2012;18:179–80. DOIPubMedGoogle Scholar
- Negredo A, de la Calle-Prieto F, Palencia-Herrejón E, Mora-Rillo M, Astray-Mochales J, Sánchez-Seco MP, et al.; Crimean Congo Hemorrhagic Fever@Madrid Working Group. Autochthonous Crimean-Congo Hemorrhagic Fever in Spain. N Engl J Med. 2017;377:154–61. DOIPubMedGoogle Scholar
- Monsalve Arteaga L, Muñoz Bellido JL, Negredo AI, García Criado J, Vieira Lista MC, Sánchez Serrano JÁ, et al. New circulation of genotype V of Crimean-Congo haemorrhagic fever virus in humans from Spain. PLoS Negl Trop Dis. 2021;15:
e0009197 . DOIPubMedGoogle Scholar - Negredo A, Sánchez-Arroyo R, Díez-Fuertes F, de Ory F, Budiño MA, Vázquez A, et al. Fatal case of Crimean-Congo hemorrhagic fever caused by reassortant virus, Spain, 2018. Emerg Infect Dis. 2021;27:1211–5. DOIPubMedGoogle Scholar
- Ruiz-Fons F, Fernández-de-Mera IG, Acevedo P, Höfle U, Vicente J, de la Fuente J, et al. Ixodid ticks parasitizing Iberian red deer (Cervus elaphus hispanicus) and European wild boar (Sus scrofa) from Spain: geographical and temporal distribution. Vet Parasitol. 2006;140:133–42. DOIPubMedGoogle Scholar
- European Centre for Disease Prevention and Control and European Food Safety Authority. Tick maps. 2023 [cited 2024 Feb 25]. https://ecdc.europa.eu/en/disease-vectors/surveillance-and-disease-data/tick-maps
- Cuadrado-Matías R, Cardoso B, Sas MA, García-Bocanegra I, Schuster I, González-Barrio D, et al. Red deer reveal spatial risks of Crimean-Congo haemorrhagic fever virus infection. Transbound Emerg Dis. 2022;69:e630–45. DOIPubMedGoogle Scholar
- Massei G, Kindberg J, Licoppe A, Gačić D, Šprem N, Kamler J, et al. Wild boar populations up, numbers of hunters down? A review of trends and implications for Europe. Pest Manag Sci. 2015;71:492–500. DOIPubMedGoogle Scholar
- Cuadrado-Matías R, Baz-Flores S, Peralbo-Moreno A, Herrero-García G, Risalde MA, Barroso P, et al. Determinants of Crimean-Congo haemorrhagic fever virus exposure dynamics in Mediterranean environments. Transbound Emerg Dis. 2022;69:3571–81. DOIPubMedGoogle Scholar
- Baz-Flores S, Herraiz C, Peralbo-Moreno A, Barral M, Arnal MC, Balseiro A, et al. Mapping the risk of exposure to Crimean-Congo haemorrhagic fever virus in the Iberian Peninsula using Eurasian wild boar (Sus scrofa) as a model. Ticks Tick Borne Dis. 2024;15:
102281 . DOIPubMedGoogle Scholar - Carrera-Faja L, Cardells J, Pailler-García L, Lizana V, Alfaro-Deval G, Espunyes J, et al. Evidence of prolonged Crimean-Congo hemorrhagic fever virus endemicity by retrospective serosurvey, eastern Spain. Emerg Infect Dis. 2022;28:1031–4. DOIPubMedGoogle Scholar
- Espunyes J, Cabezón O, Pailler-García L, Dias-Alves A, Lobato-Bailón L, Marco I, et al. Hotspot of Crimean-Congo hemorrhagic fever virus seropositivity in wildlife, northeastern Spain. Emerg Infect Dis. 2021;27:2480–4. DOIPubMedGoogle Scholar
- Triguero-Ocaña R, Laguna E, Jiménez-Ruiz S, Fernández-López J, García-Bocanegra I, Barasona JÁ, et al. The wildlife-livestock interface on extensive free-ranging pig farms in central Spain during the “montanera” period. Transbound Emerg Dis. 2021;68:2066–78. DOIPubMedGoogle Scholar
- Nigsch A. A; European Food Safety Authority. Surveillance plan proposal for early detection of zoonotic pathogens in pigs and poultry. EFSA J. 2023;20:
EN-7855 . DOIGoogle Scholar - Darwish MA, Imam IZ, Omar FM, Hoogstraal H. Results of a preliminary seroepidemiological survey for Crimean-Congo hemorrhagic fever virus in Egypt. Acta Virol. 1978;22:77.PubMedGoogle Scholar
- Mourya DT, Yadav PD, Shete AM, Gurav YK, Raut CG, Jadi RS, et al. Detection, isolation and confirmation of Crimean-Congo hemorrhagic fever virus in human, ticks and animals in Ahmadabad, India, 2010-2011. PLoS Negl Trop Dis. 2012;6:
e1653 . DOIPubMedGoogle Scholar - Moraga-Fernández A, Ruiz-Fons F, Habela MA, Royo-Hernández L, Calero-Bernal R, Gortazar C, et al. Detection of new Crimean-Congo haemorrhagic fever virus genotypes in ticks feeding on deer and wild boar, Spain. Transbound Emerg Dis. 2021;68:993–1000. DOIPubMedGoogle Scholar
- Sánchez-Seco MP, Sierra MJ, Estrada-Peña A, Valcárcel F, Molina R, de Arellano ER, et al.; Group for CCHFv Research. Widespread detection of multiple strains of Crimean-Congo hemorrhagic fever virus in ticks, Spain. Emerg Infect Dis. 2021;28:394–402. DOIPubMedGoogle Scholar
- Arenas-Montes A, García-Bocanegra I, Paniagua J, Franco JJ, Miró F, Fernández-Morente M, et al. Blood sampling by puncture in the cavernous sinus from hunted wild boar. Eur J Wildl Res. 2013;59:299–303. DOIGoogle Scholar
- Bost C, Castro-Scholten S, Sadeghi B, Cano-Terriza D, Frías M, Jiménez-Ruiz S, et al. Approaching the complexity of Crimean-Congo hemorrhagic fever virus serology: A study in swine. J Virol Methods. 2024;326:
114915 . DOIPubMedGoogle Scholar - Sas MA, Comtet L, Donnet F, Mertens M, Vatansever Z, Tordo N, et al. A novel double-antigen sandwich ELISA for the species-independent detection of Crimean-Congo hemorrhagic fever virus-specific antibodies. Antiviral Res. 2018;151:24–6. DOIPubMedGoogle Scholar
- Sas MA, Vina-Rodriguez A, Mertens M, Eiden M, Emmerich P, Chaintoutis SC, et al. A one-step multiplex real-time RT-PCR for the universal detection of all currently known CCHFV genotypes. J Virol Methods. 2018;255:38–43. DOIPubMedGoogle Scholar
- Spengler JR, Bergeron É, Rollin PE. Seroepidemiological studies of Crimean-Congo hemorrhagic fever virus in domestic and wild animals. PLoS Negl Trop Dis. 2016;10:
e0004210 . DOIPubMedGoogle Scholar - Nurettin C, Engin B, Sukru T, Munir A, Zati V, Aykut O. The seroprevalence of Crimean-Congo hemorrhagic fever in wild and domestic animals: an epidemiological update for domestic animals and first seroevidence in wild animals from Turkiye. Vet Sci. 2022;9:462. DOIPubMedGoogle Scholar
- Castillo-Contreras R, Magen L, Birtles R, Varela-Castro L, Hall JL, Conejero C, et al. Ticks on wild boar in the metropolitan area of Barcelona (Spain) are infected with spotted fever group Rickettsiae. Transbound Emerg Dis. 2022;tbed.14268.
- Pradera C, Estrada-Peña A. Hyalomma lusitanicum (Acari: Ixodidae) como potencial problema de salud pública en el área de Barcelona. Butlletí de la Institució Catalana d’Història Natural. 2022;86:111–6.
- Falcó Garí JV, López-Peña D, de la Torre J, Safont-Adsuara L, Bellido-Blasco J, Jiménez-Peydró R. Incidence of disease-transmitting ticks on the human population in the province of Castellón [in Spanish]. Presented at: XV Environmental Health Congress; May 22–24, 2019; Valencia, Spain. Rev Salud Ambient. 2019;19(Espec. Congr):135.
- Remesar S, Castro-Scholten S, Cano-Terriza D, Díaz P, Morrondo P, Jiménez-Martín D, et al. Molecular identification of zoonotic Rickettsia species in Ixodidae parasitizing wild lagomorphs from Mediterranean ecosystems. Transbound Emerg Dis. 2022;69:e992–1004. DOIPubMedGoogle Scholar
- Díaz-Cao JM, Adaszek Ł, Dzięgiel B, Paniagua J, Caballero-Gómez J, Winiarczyk S, et al. Prevalence of selected tick-borne pathogens in wild ungulates and ticks in southern Spain. Transbound Emerg Dis. 2022;69:1084–94. DOIPubMedGoogle Scholar
- Valcárcel F, González J, González MG, Sánchez M, Tercero JM, Elhachimi L, et al. Comparative ecology of Hyalomma lusitanicum and Hyalomma marginatum Koch, 1844 (Acarina: Ixodidae). Insects. 2020;11:303. DOIPubMedGoogle Scholar
- Adam IA, Mahmoud MA, Aradaib IE. A seroepidemiological survey of Crimean Congo hemorrhagic fever among cattle in North Kordufan State, Sudan. Virol J. 2013;10:178. DOIPubMedGoogle Scholar
- Phonera MC, Simuunza MC, Kainga H, Ndebe J, Chembensofu M, Chatanga E, et al. Seroprevalence and risk factors of Crimean-Congo hemorrhagic fever in cattle of smallholder farmers in central Malawi. Pathogens. 2021;10:1613. DOIPubMedGoogle Scholar
- Ciebiera O, Łopińska A, Gabryś G. Ticks on game animals in the fragmented agricultural landscape of western Poland. Parasitol Res. 2021;120:1781–8. DOIPubMedGoogle Scholar
- Atim SA, Ashraf S, Belij-Rammerstorfer S, Ademun AR, Vudriko P, Nakayiki T, et al. Risk factors for Crimean-Congo Haemorrhagic Fever (CCHF) virus exposure in farming communities in Uganda. J Infect. 2022;85:693–701. DOIPubMedGoogle Scholar
- Matthews J, Secka A, McVey DS, Dodd KA, Faburay B. Serological prevalence of Crimean-Congo hemorrhagic fever virus infection in small ruminants and cattle in the Gambia. Pathogens. 2023;12:749. DOIPubMedGoogle Scholar
- Balinandi S, von Brömssen C, Tumusiime A, Kyondo J, Kwon H, Monteil VM, et al. Serological and molecular study of Crimean-Congo Hemorrhagic Fever Virus in cattle from selected districts in Uganda. J Virol Methods. 2021;290:
114075 . DOIPubMedGoogle Scholar - Bouaicha F, Eisenbarth A, Elati K, Schulz A, Ben Smida B, Bouajila M, et al. Epidemiological investigation of Crimean-Congo haemorrhagic fever virus infection among the one-humped camels (Camelus dromedarius) in southern Tunisia. Ticks Tick Borne Dis. 2021;12:
101601 . DOIPubMedGoogle Scholar - Goletic T, Satrovic L, Softic A, Omeragic J, Goletic S, Soldo DK, et al. Serologic and molecular evidence for circulation of Crimean-Congo hemorrhagic fever virus in ticks and cattle in Bosnia and Herzegovina. Ticks Tick Borne Dis. 2022;13:
102004 . DOIPubMedGoogle Scholar - Nalca A, Whitehouse CA. Crimean-Congo hemorrhagic fever virus infection among animals. In: Ergonul, O, Whitehouse, CA, editors. Crimean-Congo hemorrhagic fever. Dordrecht (the Netherlands): Springer; 2007.
- Spengler JR, Estrada-Peña A, Garrison AR, Schmaljohn C, Spiropoulou CF, Bergeron É, et al. A chronological review of experimental infection studies of the role of wild animals and livestock in the maintenance and transmission of Crimean-Congo hemorrhagic fever virus. Antiviral Res. 2016;135:31–47. DOIPubMedGoogle Scholar
Figure
Tables
Cite This ArticleOriginal Publication Date: April 16, 2024
Table of Contents – Volume 30, Number 5—May 2024
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Javier Caballero-Gómez, Clinical Virology and Zoonoses, Maimonides Biomedical Research Institute of Cordoba (IMIBIC), Avenida Menéndez Pidal, s/n. 14004, Córdoba, Spain
Top