Volume 30, Number 7—July 2024
Dispatch
Vaccine Effectiveness against SARS-CoV-2 among Household Contacts during Omicron BA.2–Dominant Period, Japan
Cite This Article
Citation for Media
Abstract
We calculated attack rates for household contacts of COVID-19 patients during the SARS-CoV-2 Omicron BA.2–dominant period in Japan. Attack rates among household contacts without recent (<3 months) vaccination was lower for contacts of index patients with complete vaccination than for contacts of index patients without complete vaccination, demonstrating indirect vaccine effectiveness.
BA.2 is a subvariant of the SARS-CoV-2 Omicron variant. XBB.1.5 and XBB.1.16, recombinants of the BA.2.10.1 and BA.2.75 sublineages, were circulating SARS-CoV-2 variants of interest in July 2023 (1,2), but few estimates of vaccine effectiveness (VE) against infectiousness of those variants were available.
On April 20, 2022, the government of Japan required local public health centers (PHCs) to be notified of all COVID-19 patients and to implement contact tracing (3). By that date, the jurisdiction of the Itako PHC (population ≈265,000) in Ibaraki Prefecture had 13,555 confirmed COVID-19 patients (5.1% of the population) (4). Among patients with confirmed COVID-19, a total of 2.0% were hospitalized during April–May 2022.
In our previous study of the Omicron BA.1 dominant period in Itako (5), the household contact attack rate (HCAR) was lower for household contacts of index patients who were completely vaccinated than for household contacts of other index patients. However, the vaccination status of household contacts was associated with the vaccination status of the associated index patient (5). Therefore, adjustment, such as stratification for confounding by the contacts’ vaccination status, was needed for adequate evaluation of VE against infectiousness. This study aimed to assess VE against the infectiousness of the SARS-CoV-2 Omicron BA.2 variant among household contacts.
We obtained information on COVID-19 patients and household contacts reported in the jurisdiction of the Itako PHC in Ibaraki Prefecture, Japan, during April 21–30, 2022. The epidemiologic investigation and data collection procedure on COVID-19 index patients was almost the same as that described in our previous study (5). The study design was approved by the Ibaraki Prefecture Epidemiologic Research Joint Ethics Review Committee (protocol no. R3-10). We performed statistical analyses by using R version 4.4.1 (The R Foundation for Statistical Computing, https://www.r-project.org).
Genomic sequencing surveillance showed that 90% of 5,630 variants of concern sampled in Japan during April 18–May 1 were BA.2 (6). In Ibaraki, sequencing of 69 samples collected during April 23–May 13 demonstrated that 65 (94%) samples were BA.2 and 4 (6%) were BA.1.
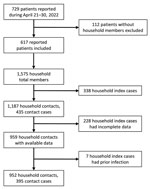
Itako PHC undertook or requested SARS-CoV-2 testing of household contacts who had any symptoms during their 5-day observational and quarantine period of a COVID-19 patient (5,7,8). Asymptomatic household contacts were not tested. During the study period, the PHC jurisdiction identified 729 confirmed COVID-19 cases (4) (Figure). After excluding 112 index cases without household members, we identified 617 infected household members, and a total of 1,575 household members, including cases and contacts. We deemed patients with the earliest symptom onset date as index cases in the household, which comprised 388 patients. Of 1,187 household contacts of the 388 index patients, we included 952 who had available data and no history of previous infection in the study. Of the 235 excluded household contacts, we excluded 7 because of a history of infection before the study period. Of the 952 contacts, 395 were infected; the overall HCAR was 41.5%.
Excluding health professionals, 82.5% of residents in Ibaraki had completed 2 COVID-19 vaccine doses, and 56.3% had received a booster dose by April 20, 2022 (9). Among initial vaccinations in Ibaraki, 81% were BNT162b2 vaccine (Pfizer-BioNTech, https://www.pfizer.com) and 19% mRNA-1273 vaccine (Moderna, https://www.modernatx.com) (5).
We stratified vaccination status on the basis of vaccination doses or recent vaccination, which we defined as receiving the last vaccine dose within 3 months. HCAR was 30.9% for contacts with 3-dose vaccination and 32.4% for contacts with recent vaccination, substantially lower than the 49.6% for contacts without complete vaccination (0–1 doses) and 46.0%, without recent vaccination (Table 1). Of the 307 contacts with 3-dose vaccination status, 272 had been vaccinated within the previous 3 months.
The HCAR for contacts of an index patient who had a 2-dose vaccination status was 37.2% and HCAR was 33.1% for contacts of index cases with a 3-dose status. Those results were substantially lower than the 46.2% for contacts of an index patient without complete vaccination status. However, the HCAR for contacts of an index patient with recent vaccination was not substantially lower than that for contacts of an index patient without recent vaccination (Table 1).
Among household contacts who had a 2-dose vaccination status, HCAR was 34.8% for contacts of index patients with complete vaccination but was 49.5% for contacts of index patients without complete vaccination status. However, among household contacts with 3-dose vaccination and without complete vaccination, the HCAR was not much lower for contacts of index patients with complete vaccination than for contacts of index patients without complete vaccination status (Table 2). The HCAR among household contacts without recent vaccination was 37.9% for contacts of index patients with complete vaccination, compared with 52.1% for contacts of index patients without complete vaccination (relative risk 0.73 [95% CI 0.61–0.87]); VE 0.27 [95% CI 0.13–0.39]) (Table 2). We obtained similar results in multivariate analyses (Appendix Table). Among household contacts with recent vaccination, HCAR was not substantially different for contacts of index patients with complete vaccination status and contacts of patients without complete vaccination status (Table 2).
Our study found the HCAR among household contacts of index SARS-CoV-2 Omicron BA.2 patients with complete vaccination was substantially lower than that among contacts of index patients without complete vaccination. In our previous study on the Omicron BA.1 variant–dominant period, the VE was 0.43 against infectiousness (5). Several other previous studies also reported VE against Omicron infectiousness (10–12); however, the vaccination doses of index patients in those studies might have been associated with the vaccination doses of their household contacts.
Among household contacts without recent vaccination in our study, HCAR was lower for contacts of index patients with complete vaccination status than for contacts of index patients without complete vaccination, a VE of 0.27. However, among household contacts with recent vaccination, we noted no major effect of vaccination completeness for index patients.
VE was greatly affected by vaccination completeness for index patients among household contacts with 2-dose vaccination but not among household contacts with 3-dose vaccination. Another study in Japan also showed relatively low VE against infectiousness among contacts with 3-dose vaccination compared with VE against infectiousness among other contacts (13).
In this study, the HCAR for contacts of an index patient with recent vaccination was not much lower than for contacts of an index patient without recent vaccination. Previous studies have reported relatively stable VE against infectiousness compared with VE against susceptibility (12,14).
The first limitation of this study is that we did not adjust for confounding factors, such as demographic data, for analyses on all household contacts. The second limitation is that we did not confirm specific variant types with genomic sequencing and different variants might have had different infection rates. Finally, our assessment of risk factors and potential confounders might be limited to the data reported to the PHCs.
In summary, our results indicate that the SARS-CoV-2 Omicron BA.2 attack rate among household contacts without recent vaccination was lower for household contacts of vaccinated index patients than for contacts of unvaccinated index patients. These results strengthen data on indirect effectiveness of COVID-19 vaccines and the value of continued vaccine campaigns to prevent severe illness among index cases and household contacts.
Dr. Ogata is the director of the Itako Public Health Center and works to evaluate data on and respond to epidemics in the center’s jurisdiction. His primary research interest is strengthening infectious disease epidemiology among public health professionals in public health centers throughout Japan.
Acknowledgment
We thank the staff of the Itako Public Health Center, Ibaraki Prefectural Institute of Public Health, and the Ibaraki Prefectural Government for their contributions to our study. We thank Editage (http://www.editage.com) for editing and reviewing this manuscript for English language.
References
- World Health Organization. Tracking SARS-CoV-2 variants. [cited 2023 Jul 17]. https://www.who.int/en/activities/tracking-SARS-CoV-2-variants
- National Institute of Infectious Diseases. Current situation of infection. 2023 Apr 19 [cited 2023 Jul 17]. https://www.niid.go.jp/niid/en/2019-ncov-e/12009-covid19-ab121st-en.html
- National Institute of Infectious Diseases, Infectious Disease Epidemiology Center. Manual for conducting active epidemiological surveillance of patients with novel coronavirus infection [cited 2023 Jul 17]. https://www.niid.go.jp/niid/en/2019-ncov-e/2484-idsc/9472-2019-ncov-02-en.html
- Ibaraki Prefectural Government. Current situation of COVID-19 patients in the prefecture [in Japanese] [cited 2023 Jul 17]. https://www.pref.ibaraki.jp/1saigai/2019-ncov/shiryouteikyou.html
- Ogata T, Tanaka H, Tanaka E, Osaki N, Noguchi E, Osaki Y, et al. Increased secondary attack rates among the household contacts of patients with the Omicron variant of the coronavirus disease 2019 in Japan. Int J Environ Res Public Health. 2022;19:8068. DOIPubMedGoogle Scholar
- Advisory Board of the Ministry of Health. Labour and Welfare. COVID-19: current situation of infection and others [in Japanese] [cited 2023 Jul 17]. https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000121431_00348.html
- Ministry of Health. Labour and Welfare. Handling of admission, discharge, and close contacts, and disclosure regarding the patients confirmed infection with B.1.1.529 strain (Omicron variant) [in Japanese] [cited 2023 Jul 17]. https://www.niph.go.jp/h-crisis/wp-content/uploads/2022/01/20220120103933_content_000881572.pdf
- Ministry of Health. Labour and Welfare. The identification of close contacts, restrictions of behavior, and implementation of contact tracing by each transmission setting based on the characteristics of the B.1.1.529 (Omicron variant) while it is the mainstream [in Japanese] [cited 2023 Jul 17]. https://www.mhlw.go.jp/content/000968056.pdf
- Ibaraki Prefectural Government. Vaccination status [in Japanese] [cited 2023 Jul 17]. https://ibaraki.stopcovid19.jp
- Lyngse FP, Kirkeby CT, Denwood M, Christiansen LE, Mølbak K, Møller CH, et al. Household transmission of SARS-CoV-2 Omicron variant of concern subvariants BA.1 and BA.2 in Denmark. Nat Commun. 2022;13:5760. DOIPubMedGoogle Scholar
- Tan ST, Kwan AT, Rodríguez-Barraquer I, Singer BJ, Park HJ, Lewnard JA, et al. Infectiousness of SARS-CoV-2 breakthrough infections and reinfections during the Omicron wave. Nat Med. 2023;29:358–65. DOIPubMedGoogle Scholar
- Mongin D, Bürgisser N, Laurie G, Schimmel G, Vu DL, Cullati S, et al.; Covid-SMC Study Group. Effect of SARS-CoV-2 prior infection and mRNA vaccination on contagiousness and susceptibility to infection. Nat Commun. 2023;14:5452. DOIPubMedGoogle Scholar
- Maeda M, Murata F, Fukuda H. Effect of COVID-19 vaccination on household transmission of SARS-CoV-2 in the Omicron era: The Vaccine Effectiveness, Networking, and Universal Safety (VENUS) study. Int J Infect Dis. 2023;134:200–6. DOIPubMedGoogle Scholar
- Braeye T, Catteau L, Brondeel R, van Loenhout JAF, Proesmans K, Cornelissen L, et al. Vaccine effectiveness against transmission of alpha, delta and omicron SARS-COV-2-infection, Belgian contact tracing, 2021-2022. Vaccine. 2023;41:3292–300. DOIPubMedGoogle Scholar
Figure
Tables
Cite This ArticleOriginal Publication Date: June 12, 2024
Table of Contents – Volume 30, Number 7—July 2024
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Tsuyoshi Ogata, Itako Public Health Center of Ibaraki Prefectural Government, Osu 1446-1, Itako, Ibaraki, 311-2422, Japan
Top