Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released.
Volume 31, Number 11—November 2025
Dispatch
Two Autochthonous Cases of Anaplasmosis, Washington, USA, 2022–2023
Suggested citation for this article
Abstract
We describe 2 cases of autochthonous human anaplasmosis in Washington, USA, where anaplasmosis has been rarely reported. Clinicians should consider anaplasmosis in the differential diagnosis for patients with compatible clinical symptoms after tick bite or time spent outdoors in an area where Ixodes pacificus ticks are present.
Anaplasmosis is a tickborne disease caused by Anaplasma phagocytophilum, a bacterium spread by some Ixodes spp. ticks (1). I. pacificus, the western black-legged tick, is the primary vector for A. phagocytophilum on the West Coast of the United States (2). Most patients with anaplasmosis experience moderate illness, including fever, malaise, headache, myalgia, nausea, vomiting, or diarrhea (3–6). The disease can progress to severe illness, with 31% of reported case-patients hospitalized, and rarely to death; the case-fatality rate is 0.3% in the United States (7). The risk for severe illness increases with advanced patient age, immunosuppression, and delayed diagnosis and treatment (6,7). Laboratory testing often shows transaminitis and cytopenias, including anemia, thrombocytopenia, and leukopenia (3–6). Peripheral blood smear tests may show morulae in granulocytes during acute illness, but that test is not a sensitive method for diagnosis (4,8). Definitive diagnosis relies on molecular testing, immunohistochemistry, or culture. Serologic testing is less specific (4).
The seasonality of human anaplasmosis cases in the United States coincides with vector activity. Nymphal Ixodes spp. ticks are active during March through early July; most anaplasmosis cases in the United States occur during May−August. A smaller peak in cases takes place during October−November, when adult Ixodes ticks are active (9).
In Washington, USA, the range of I. pacificus ticks encompasses western Washington and the eastern slopes of the Cascade mountains (10). Tick surveillance conducted by the Washington State Department of Health during 2011−2017 identified A. phagocytophilum in I. pacificus and I. spinipalpis ticks (2). Despite detection of A. phagocytophilum in ticks in the state, reports of autochthonous cases are rare but have been documented in canines with no recent travel outside of western Washington (11). We describe 2 reported human cases of autochthonous anaplasmosis in Washington.
In July 2022, an 81-year-old man (case-patient 1) visited an urgent care center with symptoms of fever (starting that day), shortness of breath, and dizziness. His medical history was notable for paroxysmal atrial fibrillation, congestive heart failure with dyspnea on exertion, pulmonary embolism, hypertension, dyslipidemia, and stroke. Bloodwork revealed thrombocytopenia (78,000 platelets/µL; reference range 140,000–400,000 platelets/µL), elevated aspartate aminotransferase (58 U/L; reference 10–35 U/L) and acute kidney injury with elevated blood urea nitrogen (32 mg/dL; reference 7–25 mg/dL) and creatinine (1.51 mg/dL; reference 0.70–1.22 mg/dL). Treating physicians discharged the patient with instructions to return for care if his symptoms worsened.
The patient sought treatment at an emergency department 2 days later for worsening shortness of breath, fatigue, weakness, and fever reaching 103°F. His platelet count had worsened to 44,000 platelets/µL, and his aspartate aminotransferase level was 86 U/L). A computed tomography angiogram identified mild cardiomegaly and trace bilateral pleural effusion but no pulmonary embolism. Computed tomography of the abdomen and pelvis revealed unremarkable results, and results of a respiratory virus panel (Biofire Respiratory 2.1 Panel, https://www.biofiredx.com) were negative. The patient received a dose of ceftriaxone and was admitted to the hospital. Consultation with infectious disease specialists prompted blood sample collection for tickborne disease testing, and physicians initiated empiric doxycycline 3 days after admission. The patient showed improvement in platelet count and liver function on that day and was discharged. Five days after discharge, the patient’s blood sample results returned positive for A. phagocytophilum by qualitative real-time PCR conducted a commercial laboratory, and physicians prescribed a continued 10-day course of doxycycline. His symptoms resolved ≈1 month after discharge.
The commercial laboratory reported the patient’s PCR results to the local health jurisdiction, who forwarded the sample to the Centers for Disease Control and Prevention (Atlanta, GA, USA), where real-time PCR and sequence analysis confirmed A. phagocytophilum. Upon interview, the patient reported no travel outside of Washington state and no tick detections or tick bites during the exposure period (5−21 days before symptom onset). Three weeks before symptom onset, the patient did visit Mason County, Washington, for several days and performed yard work in an area where a neighbor recently reported a tick bite. Public health officials presumed that location to be the patient’s likely exposure location. Environmental investigation did not begin until April 2023, because nymphal ticks are not likely to be active in August; however, limited drag sampling in April revealed no ticks for collection.
In June 2023, a woman (case-patient 2) residing in Washington began experiencing fever, back pain, neck stiffness, and headache. The woman had a history of psoriasis and hypothyroidism, managed with levothyroxine and a topical corticosteroid. Four days after symptom onset, she visited an urgent care center, where peripheral blood smear testing showed evidence of circulating, unidentified atypical cells and neutropenia, suggesting an infectious disease process or leukemia. Attending physicians discharged the patient with instructions to seek care if symptoms worsened. The woman visited an emergency department 2 days later, reporting fever, severe headache, back pain, and neck stiffness. Physicians admitted her to the hospital, where bloodwork revealed leukopenia (leukocytes 2,540/µL; reference range 4,000–12,000/µL), thrombocytopenia (platelets 96,000/µL), anemia (erythrocytes 3.5 million/µL; reference 4–5.5 million/µL), and transaminitis (alanine transaminase 691 IU/L, reference 6–60 IU/L; aspartate aminotransferase 786 IU/L, reference 5–40 IU/L; alkaline phosphatase 435 IU/L, reference 28–126 IU/L). Computed tomography showed hepatosplenomegaly but no other abnormalities, and additional serologic testing showed negative results for cytomegalovirus, hepatitis (A, B, and C), HIV, and Toxoplasma. A respiratory virus panel (VERIGENE Respiratory Pathogens Flex Test, https://us.diasorin.com) and 2 sets of blood cultures revealed negative results. The patient tested positive for group A Streptococcus on throat culture and had low-level viremia with Epstein-Barr virus, although antibody testing was negative. An infectious disease specialist consulted on the day of admission recommended an extensive work-up for viral and tickborne diseases, and attending physicians initiated a course of cefepime and doxycycline the next day. A blood sample collected the day after admission tested positive for A. phagocytophilum via real-time PCR on day 6 of hospitalization, and physicians prescribed a continuing regimen of doxycycline for 10 days. Thrombocytopenia and leukopenia resolved 4 days after starting doxycycline, and physicians discharged the patient 10 days after admission, noting gradual improvement in symptoms.
The hospital laboratory reported the real-time PCR results for this patient to officials at the local health jurisdiction, who interviewed the patient. The patient reported hiking in multiple parks in Pierce County, but no travel outside of Washington during the exposure period. No environmental investigation took place because the patient did not report a tick bite and researchers could identify no single exposure location.
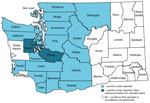
Washington is a low incidence region for tickborne diseases (2), posing a challenge for public health surveillance, prevention communication, and provider education. We charted the counties in Washington with documented I. pacificus tick presence and the suspected counties of exposure for the 2 human cases of anaplasmosis (Figure).
Clinicians should be aware of the local presence of A. phagocytophilum and the distribution of I. pacificus ticks in Washington. I. pacificus ticks can also transmit Borrelia burgdorferi sensu stricto, the causative agent of Lyme disease, and Borrelia miyamotoi, the causative agent of hard tick relapsing fever (2). Health officials have increased tick surveillance in Washington, noting increased I. pacificus ticks activity in spring and most ticks collected during March−May (2,13). To support prompt diagnosis and treatment of suspected tickborne disease among Washington residents, clinicians should consider travel history, exposure to tick habitats, seasonality in tick activity, and the patient’s clinical manifestations. More robust tick surveillance in the state could help better define vector distribution, abundance, and infection risk to inform public health prevention messaging and provider education.
Dr. Schnitzler is a zoonotic and vectorborne disease epidemiologist with the Washington State Department of Health, Office of Communicable Disease Epidemiology. Her research interests include the epidemiology and prevention of zoonotic and vectorborne diseases, using a One Health approach.
Acknowledgments
We thank the public health and environmental health staff who contributed to these case investigations at the local health jurisdictions in Whatcom, Mason, and Pierce Counties.
H.R.S., M.C., H.N.O., and E.A.D. were supported by Epidemiology and Laboratory Capacity Cooperative Agreement funding from the Centers for Disease Control and Prevention for this work. No other authors had conflicts to disclose.
References
- Chen SM, Dumler JS, Bakken JS, Walker DH. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J Clin Microbiol. 1994;32:589–95. DOIPubMedGoogle Scholar
- Dykstra EA, Oltean HN, Kangiser D, Marsden-Haug N, Rich SM, Xu G, et al. Ecology and epidemiology of tickborne pathogens, Washington, USA, 2011–2016. Emerg Infect Dis. 2020;26:648–57. DOIPubMedGoogle Scholar
- Bakken JS, Krueth J, Wilson-Nordskog C, Tilden RL, Asanovich K, Dumler JS. Clinical and laboratory characteristics of human granulocytic ehrlichiosis. JAMA. 1996;275:199–205. DOIPubMedGoogle Scholar
- Biggs HM, Behravesh CB, Bradley KK, Dahlgren FS, Drexler NA, Dumler JS, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis—United States. MMWR Recomm Rep. 2016;65:1–44. DOIPubMedGoogle Scholar
- Bakken JS, Krueth J, Wilson-Nordskog C, Tilden RL, Asanovich K, Dumler JS. Clinical and laboratory characteristics of human granulocytic ehrlichiosis. JAMA. 1996;275:199–205. DOIPubMedGoogle Scholar
- Bakken JS, Dumler S. Human granulocytic anaplasmosis.[ [viii. ]. Infect Dis Clin North Am. 2008;22:433–48, viii. DOIPubMedGoogle Scholar
- Dahlgren FS, Heitman KN, Drexler NA, Massung RF, Behravesh CB. Human granulocytic anaplasmosis in the United States from 2008 to 2012: a summary of national surveillance data. Am J Trop Med Hyg. 2015;93:66–72. DOIPubMedGoogle Scholar
- Schotthoefer AM, Meece JK, Ivacic LC, Bertz PD, Zhang K, Weiler T, et al. Comparison of a real-time PCR method with serology and blood smear analysis for diagnosis of human anaplasmosis: importance of infection time course for optimal test utilization. J Clin Microbiol. 2013;51:2147–53. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Anaplasmosis—epidemiology and statistics [cited 2023 Oct 10]. https://www.cdc.gov/anaplasmosis/hcp/statistics/index.html
- Eisen RJ, Eisen L, Beard CB. County-scale distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the continental United States. J Med Entomol. 2016;53:349–86. DOIPubMedGoogle Scholar
- Poitout FM, Shinozaki JK, Stockwell PJ, Holland CJ, Shukla SK. Genetic variants of Anaplasma phagocytophilum infecting dogs in Western Washington State. J Clin Microbiol. 2005;43:796–801. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. CDC tick surveillance data sets. December 2023 [cited 2024 Jan 4]. https://www.cdc.gov/ticks/surveillance/TickSurveillanceData.html
- Washington State Department of Health. Washington tracking network—tick data dashboard. [cited 2023 Oct 10]. https://doh.wa.gov/data-and-statistical-reports/washington-tracking-network-wtn/tick-data/tick-dashboard
Figure
Suggested citation for this article: Schnitzler H, Chan M, Nybo J, Palmer-McGee K, Doobovsky Z, Tracy I, et al. Two autochthonous cases of anaplasmosis, Washington, USA, 2022–2023. Emerg Infect Dis. 2025 Nov [date cited]. https://doi.org/10.3201/eid3111.250379
Original Publication Date: November 25, 2025
1These authors contributed equally to this article.
Table of Contents – Volume 31, Number 11—November 2025
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Hannah R. Schnitzler, Washington State Department of Health, 1610 NE 150th St, Shoreline, WA 98155, USA
Top