Volume 31, Number 2—February 2025
Dispatch
Acute Q Fever Patients Requiring Intensive Care Unit Support in Tropical Australia, 2015–2023
Abstract
Acute Q fever is classically described as a mild illness. We report 9 patients with acute Q fever in Queensland, Australia, who required intensive care unit support to survive. Clinicians should consider an acute Q fever diagnosis and its empirical treatment in critically ill persons in the appropriate clinical context.
Q fever, a zoonotic bacterial disease caused by Coxiella burnetii, has a global distribution (1). Most acute C. burnetii infections are asymptomatic or manifest as a self-limiting, nonspecific febrile illness. Respiratory and gastrointestinal symptoms can also occur, which might necessitate hospitalization, but severe, life-threatening acute disease is reported rarely (1,2). In 1 large study, only 3 (0.2%) of 1,806 patients with acute Q fever died; 2 of those deaths were because of underlying malignancy (2). We report the cases of 9 patients from Queensland in tropical Australia with laboratory-confirmed acute Q fever who required intensive care unit (ICU) support to survive their infection. The Far North Queensland Human Research Ethics Committee provided ethics approval for the study (approval no. EX/2023/QCH/95302–1707QA). Because the retrospective data were deidentified, the committee waived the requirement for informed consent.
Q fever is a notifiable disease in Australia. We used Queensland’s notifiable conditions database and electronic laboratory reporting system to identify all cases of acute Q fever in the Far North Queensland (FNQ) region during January 1, 2015–December 31, 2023. We only included cases meeting definitive laboratory criteria for acute Q fever: positive PCR or seroconversion or >4-fold increase in antibody titer to phase II antigen in paired serum samples (3). We recorded the patients’ demographic data and clinical, laboratory, and radiologic findings. We used the Charlson Comorbidity Index to quantify comorbidity; severe comorbidity was defined as a score of >5 (4). For patients with available data, we recorded the Queensland Adult Deterioration Detection System score, a vital signs–based early warning score, which was calculated when patients were first seen (5). We performed statistical analysis using Stata 18.0 (Stata, https://www.stata.com) and compared groups by using logistic regression and the Wilcoxon rank-sum, χ2, and Fisher exact tests, as appropriate.
A total of 223 cases of Q fever in the FNQ region were reported to the notifiable diseases database during the study period; 127/223 (57%) patients sought care at a hospital in the region, 105/127 (83%) were admitted as inpatients, and 9/105 (9%) were admitted to the ICU (Table 1). Eight (89%) of the 9 patients requiring ICU admission lived in a rural location. None of the 9 patients had classical occupational exposure history, and none were known to be vaccinated against Q fever; 8/9 (89%) reported close contact with animals. Eight (89%) of 9 were >50 years of age, but only 1 (11%) had severe comorbidity. Only 1/9 (11%) was first seen within 7 days of symptom onset, but 7/9 (78%) had been prescribed antimicrobial drug therapy with activity against C. burnetii for >24 hours before their ICU admission. One otherwise healthy 55-year-old woman had received doxycycline for 4 days before her ICU admission (Appendix Table 1).
The small sample size and retrospective nature of the study precluded detailed statistical analysis; however, patients requiring ICU care were more likely to have multiorgan involvement (odds ratio [OR] 5.42 [95% CI 1.21–24.31]; p = 0.03), an abnormal chest radiograph (OR 4.15 [95% CI 1.02–16.80]; p = 0.046), and an elevated early warning score (OR 5.42 [95% CI 1.21–24.31]; p = 0.03) when they were first seen (Tables 1, 2). Testing for serum antiphospholipid antibodies was performed for only 1 ICU patient (case no. 5) (Appendix); the result was positive. Three patients not requiring ICU care had serum samples tested for antiphospholipid antibodies; 1 result was negative, and 2 results were borderline positive.
The actual diagnosis of Q fever in the 9 patients requiring ICU admission was often delayed or even retrospective. Initial serologic results suggested acute Q fever in only 3/9 (33%) patients; those results were negative for 5/9 (55%) and suggested previous C. burnetii infection in 1/9 (11%) (Appendix Table 2). Serum PCR was positive in every case that was tested with that method; however, access to those PCR results was often delayed because testing was performed by the statewide reference laboratory, which was 1,390 km away. Indeed, 2 ICU patients were discharged from the hospital before their Q fever diagnosis was confirmed, and both patients received less than the recommended 14 days of antimicrobial drug therapy (7 and 10 days) (6).
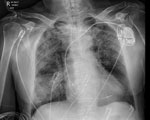
The 9 patients were in the ICU for a median of 3 (interquartile range 2–5) days; 6/9 (67%) required vasopressor support for hypotension and 1/9 (11%) required mechanical ventilation (Figure), whereas 2/9 (22%) needed no organ support but required monitoring of multiorgan dysfunction (Appendix Table 1). No patient admitted to ICU required renal replacement therapy. Indeed, for a critically ill population, the patients’ renal function was remarkably preserved; the highest recorded serum creatinine in any of the 9 patients during their hospitalization was 123 µmol/L (Appendix Table 3).
Patients requiring ICU admission spent a median of 11 (interquartile range 8–18) days in the hospital. All 9 ICU patients survived to hospital discharge, and none have subsequently received a diagnosis of chronic Q fever, although follow-up serologic testing has been performed for only 4/9 (44%) (Appendix). One patient required the insertion of a permanent pacemaker for atrioventricular block 8 months after his hospital admission, although this need was considered to be unrelated to his C. burnetii infection.
Acute Q fever is classically thought to be a mild illness; however, 9/223 (4%) patients with a confirmed acute infection in the FNQ region of Australia required ICU care to survive their infection. Severe disease in those 9 patients might be explained by a delay in seeking medical care and in appropriate antimicrobial drug therapy; only 1 patient sought care within 7 days of symptom onset, and only 4 received antimicrobial drugs with activity against C. burnetii when they were first seen. The delay in effective therapy was partly explained by a lack of timely access to PCR results, which might have expedited initiation of targeted antimicrobial drug therapy and prevented some of the patients’ subsequent deteriorations.
Acute Q fever can be life-threatening. Its complications include severe pneumonia, hepatitis, meningoencephalitis, and myocarditis (1); however, hypotension is rarely reported. It is, therefore, notable that 6 patients in this series required vasopressor support. We hypothesize that this hypotension was distributive and caused by sepsis because it responded relatively promptly to fluid resuscitation and antimicrobial drug therapy; vasopressor support was usually required for <72 hours (7). Severe disease and hypotension have not been a feature of large case series in Australia, although the clinical descriptions in those studies were frequently not detailed (8–10).
There is growing recognition of marked geographic variation in the clinical phenotype of acute Q fever, which might be explained by variation in lipopolysaccharide expression in different C. burnetii strains (1,11). Strain variation might, at least partly, explain the findings in our cohort. The presence of antiphospholipid antibodies during acute Q fever has also been associated with a complicated disease course (2) and were identified in the only patient admitted to ICU who had serum tested for antiphospholipid antibodies; this testing will now be performed routinely at our hospital. Expanded use of PCR to test for C. burnetii during the study period (available since 2016) might also have contributed to greater recognition of the severe clinical phenotype described in this cohort (Appendix Figures 1, 2).
In conclusion, acute Q fever can cause life-threatening disease in otherwise healthy persons, and the clinical phenotype can evolve even after effective antimicrobial drug therapy begins. PCR is a far more sensitive diagnostic test than serology during early C. burnetii infection, and a positive result enables prompt, potentially lifesaving therapy and enhanced follow-up to identify chronic disease.
Dr. Price is an infectious diseases registrar at the Royal Hobart Hospital in Hobart, Tasmania, Australia. His research interests include Q fever and infections in immunocompromised patients.
References
- Eldin C, Mélenotte C, Mediannikov O, Ghigo E, Million M, Edouard S, et al. From Q fever to Coxiella burnetii infection: a paradigm change. Clin Microbiol Rev. 2017;30:115–90. DOIPubMedGoogle Scholar
- Melenotte C, Protopopescu C, Million M, Edouard S, Carrieri MP, Eldin C, et al. Clinical features and complications of Coxiella burnetii infections from the French National Reference Center for Q fever. JAMA Netw Open. 2018;1:
e181580 . DOIPubMedGoogle Scholar - Australian Government, Department of Health and Aged Care. Q fever—laboratory case definition. 2017 [cited 2024 Feb 20]. https://www.health.gov.au/resources/publications/q-fever-laboratory-case-definition
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. DOIPubMedGoogle Scholar
- Campbell V, Conway R, Carey K, Tran K, Visser A, Gifford S, et al. Predicting clinical deterioration with Q-ADDS compared to NEWS, Between the Flags, and eCART track and trigger tools. Resuscitation. 2020;153:28–34. DOIPubMedGoogle Scholar
- Q fever. In: Therapeutic Guidelines. Melbourne: Therapeutic Guidelines Limited; 2019 [cited 2024 Mar 1]. https://www.tg.org.au
- Gyawali B, Ramakrishna K, Dhamoon AS. Sepsis: The evolution in definition, pathophysiology, and management. SAGE Open Med. 2019;7:
2050312119835043 . DOIPubMedGoogle Scholar - Derrick EH. The course of infection with Coxiella burneti. Med J Aust. 1973;1:1051–7. DOIPubMedGoogle Scholar
- Spelman DW. Q fever: a study of 111 consecutive cases. Med J Aust. 1982;1:547–8, 551, 553. DOIPubMedGoogle Scholar
- Graves SR, Islam A. Endemic Q fever in New South Wales, Australia: a case series (2005–2013). Am J Trop Med Hyg. 2016;95:55–9. DOIPubMedGoogle Scholar
- Eldin C, Mahamat A, Demar M, Abboud P, Djossou F, Raoult D. Q fever in French Guiana. Am J Trop Med Hyg. 2014;91:771–6. DOIPubMedGoogle Scholar
Figure
Tables
Cite This ArticleOriginal Publication Date: January 14, 2025
1Current affiliation: Royal Hobart Hospital, Hobart, Tasmania, Australia.
Table of Contents – Volume 31, Number 2—February 2025
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Josh Hanson, The Kirby Institute, Wallace Wurth Building, University of New South Wales, Kensington, NSW 2052, Australia
Top