Volume 31, Number 2—February 2025
Research Letter
Bayou Hantavirus Cardiopulmonary Syndrome, Louisiana, USA, 2022–2023
Cite This Article
Citation for Media
Abstract
During 2020–2023, we sequenced Bayou virus from 2 patients in Louisiana, USA, with hantavirus cardiopulmonary syndrome. Direct virus sequencing demonstrated an inferred evolutionary relationship to previous cases. Our findings demonstrate that separate virus spillovers cause isolated cases and probable wide distribution of Bayou hantavirus in rodents across Louisiana.
Hantavirus cardiopulmonary syndrome (HCPS) is a rodentborne zoonotic disease caused by infection with New World hantaviruses located predominantly in the Americas (1). HCPS is an acute febrile illness that can rapidly progress to acute respiratory distress syndrome and death; cases with fever but no respiratory involvement have also been identified (2). During 1993–2021, the hantavirus mortality rate in the United States was 35% (3). Disease is acquired after exposure to rodents, through inhalation of aerosolized virus from rodent urine and feces and, less frequently, from rodent bites (4). In the United States, 5 New World hantaviruses cause human disease (Table) (5,6).
Oryzomys palustris marsh rice rats are native to the southeastern United States, and Bayou virus–infected rodents have been identified in Texas, Louisiana, Georgia, and South Carolina (7,8). Only 7 human cases of Bayou virus infection have been reported, 5 in Texas and 2 in Louisiana, 1 of which was fatal (2,4,5). During 1993–2021, Louisiana reported 7 hantavirus cases; however, virus sequence information is available for only 1993 and 2013 cases (4,6). We describe 2 unrelated cases of Bayou HCPS from Louisiana reported in 2022–2023.
Patient 1 was a 66-year-old man with a medical history of tobacco use disorder who sought care at an emergency department after 4 days of chest pain, weakness, nausea, cough, and shortness of breath (9). Laboratory values indicated hemoconcentration, mildly elevated creatinine level, elevated lactate dehydrogenase level, and thrombocytopenia. Chest radiographs were concerning for bilateral infiltrates, and chest computed tomography (CT) showed small pleural effusions with patchy ground-glass opacities. At the time of arrival, the patient’s oxygen saturation was 91% with bilevel positive airway pressure and his oxygen requirements quickly escalated. His blood oxygen level decreased, and he was intubated. Laboratory analyses were notable for leukocytosis, worsening thrombocytopenia, and a granulocytic left shift; chest radiography showed worsening opacities. The patient had bilateral femoral artery clots and widespread petechiae, and he died 4 days after admission. Hantavirus serologic testing of samples collected before death were posthumously positive for IgM and negative for IgG. We could not obtain exposure information.
Patient 2 was a 56-year-old man with no relevant medical history. He experienced a syncopal episode preceded by a 1-week history of fever, cough, shortness of breath, malaise, diarrhea, and vomiting. At the time of arrival at the emergency department, he experienced a second syncopal episode. He had visited the emergency department once for this illness, which was diagnosed as a stomach virus. At admission, he was hypotensive with thrombocytopenia, leukocytosis, mildly elevated liver enzymes, elevated creatinine, and elevated lactate dehydrogenase level. Chest radiography was suggestive of bronchitis with pulmonary edema, and CT showed moderate interstitial pulmonary edema. Patient 2 was transferred to the intensive care unit for septic shock, complicated by thrombocytopenia, acute renal failure, and metabolic acidosis. Because his respiratory status deteriorated, bilevel positive airway pressure was administered, and metabolic encephalopathy developed. Subsequent CT showed bilateral pleural effusions and partial encapsulation of the left lower lung with left-sided pleural effusion. Thrombocytopenia worsened, and leukocytosis and creatinine level increased. Hemodialysis was started, and steroids and antimicrobial drugs were administered. The patient’s signs/symptoms gradually resolved, and he was discharged 15 days after admission. Hantavirus infection was confirmed by the presence of hantavirus-reactive IgM (IgG-negative) in a specimen collected 7 days after symptom onset; no subsequent specimens were collected. During a follow-up interview, the patient reported having cleaned out an uninhabited trailer during the 2–3 weeks before symptom onset, including tearing up carpets and insulation and working under the trailer without proper personal protective equipment.
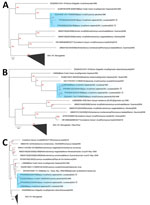
Using the Altona hantavirus quantitative reverse transcription PCR (https://altona-diagnostics.com), we determined that virus cycle thresholds were 28.4 for patient 1 and 28.8 for patient 2. We generated complete Bayou virus large (L), medium (M), and small (S) segments directly from patient serum by using the RNA Exome Library Preparation unbiased sequencing method (Illumina, https://www.illumina.com) with pan-hantavirus enrichment oligonucleotides, followed by de novo assembly and reference mapping. L segment sequences from patients 1 (GenBank accession no. PP639088) and 2 (GenBank accession no. PP639090) were closely related to a rodentborne Bayou virus collected in 1996 (Figure, panel A). The M and S segments from both patients also clustered on Bayou virus-specific clades (Figure, panels B, C). M and S segments from both patients were more closely related to historic Bayou sequences than to each other; demonstrating that separate zoonotic spillovers probably caused disease (Figure, panels B, C). Patient 1 M and S segments (GenBank accession nos. PP639086 and PP639087) were related to Bayou sequences from O. palustris rats collected in 1996 from Galveston, Texas, whereas patient 2 M and S segments (GenBank accession nos. PP639091 and PP639089) were associated with a fatal Bayou virus case from northeastern Louisiana in 1993.
For both patients, hantavirus infection was initially exhibited by nonspecific signs/symptoms and quickly progressed to severe disease. Increasing surveillance efforts and clinician education, along with implementing the hantavirus 5-point screening tool, could improve rapid diagnostics during indistinguishable disease manifestation (10).
Ms. Ortega is an epidemiologist at the Louisiana Department of Health. Her research interests include vectorborne diseases and zoonotic infections.
Acknowledgments
We thank Angie Orellana, Leslie Arceneaux, and the Louisiana Department of Health Infectious Disease Epidemiology regional surveillance teams for their work in these investigations.
All authors declare no competing interests.
References
- Jonsson CB, Figueiredo LT, Vapalahti O. A global perspective on hantavirus ecology, epidemiology, and disease. Clin Microbiol Rev. 2010;23:412–41. DOIPubMedGoogle Scholar
- National Notifiable Diseases Surveillance System. Hantavirus pulmonary syndrome (HPS) 2015 case definition [cited 2024 Jul 3]. https://ndc.services.cdc.gov/case-definitions/hantavirus-pulmonary-syndrome-2015
- Centers for Disease Control and Prevention. Reported cases of hantavirus disease [cited 2024 Jul 3]. https://www.cdc.gov/hantavirus/data-research/cases/index.html
- Knust B, Rollin PE. Twenty-year summary of surveillance for human hantavirus infections, United States. Emerg Infect Dis. 2013;19:1934–7. DOIPubMedGoogle Scholar
- Whitmer SLM, Whitesell A, Mobley M, Talundzic E, Shedroff E, Cossaboom CM, et al. Human Orthohantavirus disease prevalence and genotype distribution in the U.S., 2008-2020: a retrospective observational study. Lancet Reg Health Am. 2024;37:
100836 . DOIPubMedGoogle Scholar - Louisiana Office of Public Health, Infectious Disease Epidemiology Section. Hantavirus infection (including pulmonary syndrome) [cited 2025 Jan 16]. https://ldh.la.gov/assets/oph/Center-PHCH/Center-CH/infectious-epi/Annuals/2021/Hantavirus_LaIDAnnual.pdf
- Ksiazek TG, Nichol ST, Mills JN, Groves MG, Wozniak A, McAdams S, et al. Isolation, genetic diversity, and geographic distribution of Bayou virus (Bunyaviridae: hantavirus). Am J Trop Med Hyg. 1997;57:445–8. DOIPubMedGoogle Scholar
- Torrez-Martinez N, Bharadwaj M, Goade D, Delury J, Moran P, Hicks B, et al. Bayou virus-associated hantavirus pulmonary syndrome in Eastern Texas: identification of the rice rat, Oryzomys palustris, as reservoir host. Emerg Infect Dis. 1998;4:105–11. DOIPubMedGoogle Scholar
- Hennig J, Rosson J, Curry K. 551: A complex case of hantavirus pulmonary syndrome and hemorrhagic fever with renal syndrome. Crit Care Med. 2023;52:S249. DOIGoogle Scholar
- Koster F, Foucar K, Hjelle B, Scott A, Chong YY, Larson R, et al. Rapid presumptive diagnosis of hantavirus cardiopulmonary syndrome by peripheral blood smear review. Am J Clin Pathol. 2001;116:665–72. DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleOriginal Publication Date: January 22, 2025
Table of Contents – Volume 31, Number 2—February 2025
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Shannon Whitmer, Centers for Disease Control and Prevention, 1600 Clifton Rd NE, Mailstop H18-SSB, Atlanta, GA 30329-4018, USA
Top