Volume 31, Number 3—March 2025
Dispatch
Tsukamurella tyrosinosolvens Respiratory Infection in Immunocompetent Man
Abstract
Tsukamurella spp. are an infrequent and underdiagnosed cause of bacterial respiratory infection, usually occurring in patients with structural lung disease or immune compromise. We describe T. tyrosinosolvens respiratory infection in a patient in Australia without structural lung disease or known immune deficiency. The patient was successfully treated with oral ciprofloxacin and clarithromycin.
Tsukamurella spp. are variably or weakly acid-fast, gram-positive, non–spore-forming, obligate aerobic actinomycetes and are typically isolated from water, soil, and other terrestrial samples. Tsukamurella infections are rare but, when reported, are often associated with indwelling vascular or peritoneal catheters (1). Tsukamurella spp. also have been described as causative agents in respiratory infection, meningitis, keratitis, cutaneous infection, and acute otitis media (1,2). Nonpathogenic respiratory colonization by Tsukamurella spp. also has been described (1). Tsukamurella respiratory infection probably is underidentified because of clinical, radiologic, and morphologic similarities with related, more common organisms such as Mycobacterium tuberculosis. The advent of DNA and RNA sequencing techniques and matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry has enabled an increase in diagnosis of Tsukamurella infection (2,3).
Tsukamurella spp. respiratory infection might be clinically indistinguishable from pulmonary tuberculosis (TB); symptoms include cough, hemoptysis, and weight loss (1). Current understanding of risk factors is limited; however, 38% of previously described cases were associated with immune compromise and 69% with underlying structural lung disease (1,4–13) (Appendix). Tsukamurella pulmonary co-infection with other aerobic actinomycetes also has been described (13).
Because reported cases are rare, awareness of Tsukamurella spp. infection among clinicians is limited, and evidence to guide empiric management is scarce. We describe a case of T. tyrosinosolvens respiratory infection in an apparently immunocompetent patient in Australia without underlying structural lung disease.
A 25-year-old man, 3 years postmigration from India, was referred to our hospital with right apical nodular opacities on chest radiograph performed during routine migrant screening, which were presumed to be attributable to TB. He was asymptomatic, with no cough, dyspnea, fevers, night sweats, or recent weight loss. The patient had no remarkable medical history and did not take medications regularly. He reported no smoking or recreational drug use. He had no history of immune deficits such as HIV infection, malignancy, stem cell or solid organ transplant, steroid or other immunosuppressive drug use, diabetes mellitus, alcoholism, renal failure, liver failure, or previous splenectomy. He worked in manufacturing and had exposure to diesel engines and reported no exposure to soil, animals, or wastewater.
At initial assessment, the patient was afebrile and had no focal respiratory signs. Initial blood testing results were unremarkable; C-reactive protein and leukocyte counts were within reference ranges. Results of assay tests for TB and HIV were negative. Sputum specimens collected on 3 consecutive days were smear-negative for acid-fast bacilli (AFB) with auramine-rhodamine stain.
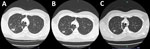
Computed tomography (CT) of the chest showed variable-sized pulmonary nodules in the right lung apex and surrounding tree-in-bud changes (Figure). CT also identified 2 cavities; the larger cavity measured 25 mm and contained an air-fluid level. The remaining lung was clear. Cultures of all 3 sputum samples indicated Tsukamurella spp. most closely related to T. tyrosinosolvens. We performed species identification through targeted Sanger DNA sequencing of a 550-bp fragment of the 16S rRNA gene, followed by BLAST analysis (https://blast.ncbi.nlm.nih.gov) to determine the most closely matched species. Given radiologic findings consistent with active infection and repeated isolation of Tsukamurella spp., we decided to treat empirically with ciprofloxacin and clarithromycin (both 500 mg 2/d).
Subsequent sensitivity testing through broth microdilution demonstrated susceptibility to a broad range of antimicrobial drugs, intermediate susceptibility to doxycycline, and resistance to amoxicillin/clavulanic acid and tobramycin (Table). Given the lack of clinical data to support development of validated MIC breakpoints for Tsukamurella spp., we interpreted susceptibilities by using MIC breakpoints for Nocardia spp. by Clinical and Laboratory Standards Institute guidelines (14). Two further sputum samples collected 1 month later also were AFB smear-negative; however, we cultured Mycobacterium fortuitum from a single specimen, which was not considered causative or otherwise clinically important.
After 3 months of treatment, the patient remained asymptomatic and reported no adverse events associated with treatment. Repeat CT showed interval reduction of the cavitating lesion from 25 to 17 mm (Figure). Treatment was continued for another 3 months, at which time the nodules had further reduced in size; the cavitating lesion measured 10 mm (Figure). After 6 months of macrolide and fluoroquinolone therapy, treatment was stopped. We noted no radiologic signs of infection recurrence on repeat CT at 6 months after treatment cessation.
We describe a case of T. tyrosinosolvens infection as a cause of cavitating respiratory disease in an immunocompetent and otherwise healthy young man. This case challenges the characterization of Tsukamurella spp. as opportunistic pathogens and should raise awareness of Tsukamurella respiratory infection. Although Tsukamurella pulmonary infection is rare, the number of reports is increasing, and most cases have been published within the last decade (Appendix). The emergence of Tsukamurella bacteria as a cause of human infection probably reflects advances in laboratory methods and increased recognition of a previously misdiagnosed disease. Although the true prevalence remains unclear, epidemiologic studies in China indicated that 1% of presumed nontuberculous Mycobacteria respiratory samples were Tsukamurella spp. when they were retrospectively analyzed using molecular methods (15).
Underrecognition occurs for several reasons. Lack of awareness among clinicians and the practice of treating AFB culture-positive infection as presumed TB in some clinical settings contribute to misdiagnosis (2,7). Because Tsukamurella spp. respiratory infection might be clinically, radiologically, and morphologically indistinguishable from pulmonary TB and might also respond to treatment with first-line TB therapy, the risk for misdiagnosis is high in the absence of microbiologic confirmation. A broader differential including other aerobic actinomycetes could be beneficial, especially in patients not responding to initial therapy. Tsukamurella resistance to first-line TB treatment agents has been described (2). Misdiagnosis has been shown to lead to excess disease and death in some case reports (7) and can also lead to use of unnecessary, toxicity-prone TB treatment regimens.
Microbiologic diagnosis often is resource-intensive. Standard laboratory phenotypic and biochemical methods might be inadequate to distinguish Tsukamurella from other aerobic actinomycetes. The advent of MALDI-TOF mass spectrometry and molecular techniques such as 16S rRNA and DNA sequencing have enabled accurate identification of Tsukamurella genus (2). Although 16S sequencing is an effective technique for identifying Tsukamurella genus, it often is insufficient to achieve species-level identification because of the high genetic conservation between species and lack of sequences available on public databases for comparison. Sequencing of additional housekeeping genes (e.g., groEL, secA, and rpoB) might be necessary for Tsukamurella species identification. Although some literature describes low diagnostic accuracy of Tsukamurella spp. with MALDI-TOF mass spectrometry, recent database improvements have demonstrated species-level identification with 98% accuracy (3). Therefore MALDI-TOF mass spectrometry might be an efficacious and cost-effective diagnostic method compared with DNA and RNA sequencing techniques. Unfortunately, those methods might not be routinely available outside metropolitan clinical microbiology and reference laboratories and can be impractical in settings with high TB prevalence.
Even once diagnosis is made, evidence to guide antibiotic choice and duration of therapy is scarce. Empiric regimens are based on previous case reports, and duration must be guided by clinical response. In the absence of validated MIC breakpoints, correlation between in vivo and in vitro sensitivities might be poor. Current Clinical and Laboratory Standards Institute guidelines provide tentative breakpoints for interpreting Tsukamurella spp. susceptibility testing on the basis of Nocardia spp. breakpoints (14); however, patients should be monitored to ensure appropriate clinical response.
Dr. Clifford is a medical registrar at Western Health. His primary research interests include infectious disease and global health.
Acknowledgments
We thank the staff at Footscray Hospital for their tireless commitment to patient care.
The patient has provided consent for the publication of this case report.
We declare no conflict of interest, and the authors received no financial support for this work.
References
- Safaei S, Fatahi-Bafghi M, Pouresmaeil O. Role of Tsukamurella species in human infections: first literature review. New Microbes New Infect. 2017;22:6–12. DOIPubMedGoogle Scholar
- Yu S, Ding X, Hua K, Zhu H, Zhang Q, Song X, et al. Systematic investigation of the emerging pathogen of Tsukamurella species in a Chinese tertiary teaching hospital. Microbiol Spectr. 2023;11:
e0164423 . DOIPubMedGoogle Scholar - Teng JLL, Tang Y, Wong SSY, Fong JYH, Zhao Z, Wong CP, et al. MALDI-TOF MS for identification of Tsukamurella species: Tsukamurella tyrosinosolvens as the predominant species associated with ocular infections. Emerg Microbes Infect. 2018;7:80. DOIPubMedGoogle Scholar
- Romano L, Spanu T, Calista F, Zappacosta B, Mignogna S, Sali M, et al. Tsukamurella tyrosinosolvens and Rhizobium radiobacter sepsis presenting with septic pulmonary emboli. Clin Microbiol Infect. 2011;17:1049–52. DOIPubMedGoogle Scholar
- Yang L, Cao Y, Dan Z, Wang Z, Wang X. Community-acquired Tsukamurella pneumonia in a young immunocompetent adult: a case misdiagnosed as pulmonary tuberculosis and literature review. Postgrad Med. 2017;129:563–6. DOIPubMedGoogle Scholar
- Sandhu JE, Ariyaratnam J. D Trandafirescu T. Nocardia Tsukamurella: an atypical pneumonia. Chest. 2022;162(Supplement):A561. DOIGoogle Scholar
- Liu X, Shi J, Wang X, Chen Y, Zheng L. Tsukamurella pneumonia misdiagnosed as pulmonary tuberculosis. Lancet Infect Dis. 2022;22:1090. DOIPubMedGoogle Scholar
- Kuge T, Fukushima K, Matsumoto Y, Saito H, Abe Y, Akiba E, et al. Chronic pulmonary disease caused by Tsukamurella toyonakaense. Emerg Infect Dis. 2022;28:1437–41. DOIPubMedGoogle Scholar
- Gotoh K, Mayura IPB, Hagiya H, Obata K, Ogawa T, Iio K, et al. Septic pulmonary emboli caused by Tsukamurella inchonensis: A case report. J Infect Chemother. 2021;27:369–72. DOIPubMedGoogle Scholar
- Swier R, Jakharia KK. Acid-fast bacteria in bronchiectasis: if the glass slipper does not fit, non-TB mycobacteria, consider Tsukamurella. Chest. 2022;162:A573. DOIGoogle Scholar
- Manek G, Shah N, Krishnan AM, Anthony P. A case of Tsukamurella pulmonis masquerading as pleural TB in an immunocompetent patient. Chest. 2019;156(Supplement):A252. DOIGoogle Scholar
- Akkineni S, Calderon Candelario RA, Mirsaeidi M. Acute COPD exacerbation associated with a rare pathogen: a case of Tsukamurella pulmonis. Am J Respir Crit Care Med. 2019;199:A6448.
- Rao S, Paz M, Nugent K. Tsukamurella and Mycobacterium tuberculosis pneumonia co-infection. Am J Med Sci. 2023;365:S163-S.
- Clinical and Laboratory Standards Institute. Susceptibility testing of mycobacteria, Nocardiae, and other aerobic actinomycetes. 2nd edition. Wayne (PA): The Institute; 2011 [cited 2024 Sep 24]. https://www.ncbi.nlm.nih.gov/books/NBK544374
- Sun Q, Yan J, Liao X, Wang C, Wang C, Jiang G, et al. Trends and species diversity of non-tuberculous Mycobacteria isolated from respiratory samples in Northern China, 2014-2021. Front Public Health. 2022;10:
923968 . DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleOriginal Publication Date: February 21, 2025
Table of Contents – Volume 31, Number 3—March 2025
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Aidan Clifford, Sunshine Hospital, 176 Furlong Rd, St Albans, VIC 3021, Australia
Top