Volume 31, Number 4—April 2025
Dispatch
Onset of Alpha-Gal Syndrome after Tick Bite, Washington, USA
Cite This Article
Citation for Media
Abstract
We describe a case of alpha-gal syndrome (AGS) in a resident of Washington, USA, after local Ixodes pacificus tick bites, which were associated with IgE increases after diagnosis. AGS should be considered a potential cause of anaphylactic and allergic reactions in persons with tick exposures, regardless of geographic residence.
Alpha-gal syndrome (AGS) is an allergy characterized by IgE-mediated hypersensitivity to galactose-α-1,3-galactose (α-gal), a disaccharide found in most nonprimate mammalian tissue (1). Epidemiologic studies have linked tick bites to AGS development (2–5). The number of documented AGS cases in the United States has increased since 2010, predominantly in the southern, midwestern, and mid-Atlantic regions (6). An association between lone star ticks (Amblyomma americanum) and α-gal sensitization has been well described. However, recent studies have identified cases outside the known range of this tick, and α-gal has been identified in the saliva of other tick species, including Ixodes scapularis (6,7). Evidence linking AGS to bites from ticks found in the western United States is lacking. We describe a case of AGS in a female resident of Washington, USA, who had symptom onset after tick bites in Washington and had no exposure to areas with known lone star ticks within the previous 30 years.
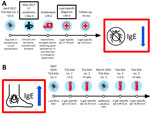
In May 2017, a 61-year-old female wildlife biologist reported onset of diffuse urticaria and lip edema (day 0) (Figure). She called a consulting nurse and was advised to take ranitidine and diphenhydramine; symptoms resolved within 24 hours. On day 29, she had itching in her groin and noted urticaria on her back. Within a few minutes, she reported that her tongue felt swollen; she called 911, noting difficulty speaking. Paramedics documented diffuse urticaria, tongue edema, dysphonia, inspiratory wheezing, systolic blood pressure at 80 mm Hg, and heart rate at 120 beats/min. Epinephrine (0.3 mg) was administered intramuscularly (IM) for anaphylaxis, and mild improvement was observed. However, within several minutes, her clinical course worsened, and notable progression of tongue edema, throat tightness, and onset of tunnel vision occurred. A second dose of epinephrine (0.3 mg IM) was administered. While enroute to the emergency department, symptoms progressed, and she was given methylprednisolone (125 mg IM) and nebulized racemic epinephrine. Upon arrival at the emergency department, her symptoms were improving, and examination revealed mild urticaria and blood pressure of 117/57 mm Hg. She was discharged after a 4-hour observation and prescribed prednisone, famotidine, diphenhydramine, and an epinephrine autoinjector.
No unusual activities or exposures immediately preceding either episode (day 0 or 29) were documented in the initial exposure history, although she was outside before both reactions. She had no known stings immediately preceding symptoms and no previous illnesses. She was not taking routine medications and reported no previous episodes of hives or swelling. During a later interview, the patient recalled that ≈1 month before the initial episode (day −32), she had walked her dog in a wooded area and had itching on her back. She had inspected the area and found a nonengorged tick embedded in her left shoulder, estimating ≈12 hours of attachment. She had attempted to remove the tick, but some mouthpart material remained; the area subsequently became painful and red, enlarging to a size greater than the palm of her hand. She sought care at the emergency department the same day, where mouthparts were removed and doxycycline was prescribed.
A detailed food history indicated the patient had eaten beef tacos 6 hours before the first episode of angioedema (day 0) and consumed both pork sausage 9 hours and a ham sandwich 4 hours before the second reaction (day 29); she reported rarely eating red meat. She recalled no other tick bites since working in Wyoming in the 1980s. Her lifetime travel history included brief visits to Florida and Maryland (lone star tick populations expected) >30 years before, as well as a trip to northern Illinois in 2016 along with a road trip back to Washington through Minnesota, South Dakota, Montana, and Idaho (lone star tick populations not established).
On day 40, an allergist (W.K.B.) evaluated her for presumed idiopathic anaphylaxis. The following laboratory results were within reference ranges: complete blood cell count, Chronic Urticaria Index (4.8 units), serum protein electrophoresis, bee venom–specific IgE (<1 ng/mL for 6 stinging insect venoms), tryptase (5.4 ng/mL), and 24-hour N-methylhistamine (129 μg/g creatinine). However, her α-gal IgE was positive at 27.40 kU/L (reference range <0.1 kU/L). AGS was diagnosed, and she was told to strictly avoid consuming mammalian meat and cautioned about consuming milk and gelatin. She subsequently avoided meat and gelatin continuously and had no further allergic episodes. Within 6 months, her α-gal IgE had fallen from 27.40 kU/L to 6.91 kU/L in the absence of intervening tick bites.
In April 2020, the patient reported a second tick bite that occurred ≈1 mile from the original bite in 2017. The tick was embedded on her upper back for ≈10 hours before removal. Her cutaneous reaction to this bite site was similar to that of the previous episode (redness, heat, and extreme itchiness), and the bite area measured ≈1.5 inches in diameter. Three days after this second bite, her α-gal IgE level was 0.72 kU/L, increasing to 20.20 kU/L 4 weeks later. She submitted the tick to the Washington State Department of Health through the passive tick surveillance program, where it was identified as an adult female I. pacificus tick. Had the patient not switched to a vegetarian diet, clinical experience suggests that the rise in IgE titer might have increased her chances of having an allergic reaction after red meat consumption (8).
In March 2022, the patient reported a third tick bite on the inside of her right knee. Tick attachment was estimated to be <1 hour. She experienced a similar itchy reaction at the bite site. Clinical follow-up documented another IgE titer increase, from 0.89 kU/L initially to 18.80 kU/L 4 weeks later. She also submitted this tick to the Washington State Department of Health, where it was identified as an adult female I. pacificus tick. The patient included details regarding her symptoms and AGS diagnosis with this tick submission, and the Washington State Department of Health initiated a public health investigation, which included a detailed exposure interview.
The habitat of the I. pacificus tick is known to extend along the Pacific Coast of the United States; it is commonly found in northern California, Oregon, and Washington and the coast of British Columbia, Canada (9). Although the genus Ixodes has been associated with AGS in Australia (10), Scandinavia (11), and Europe (12–14), it has yet to be conclusively linked with AGS in the United States. Commercial laboratories have documented α-gal–specific IgE in samples from patients residing outside of the known range of lone star ticks, including in several western US states (6,15). Although those persons might have traveled, the possibility of local exposure cannot be excluded. However, observed AGS incidence in those areas appears low relative to the high number of reported Ixodes tick bites. Therefore, those findings might reflect a limited role for tick species other than A. americanum in AGS development in the United States, which is further supported through review of geographic distribution of suspected cases and range of A. amblyomma ticks (6,15).
We provide evidence that bites from I. pacificus ticks might stimulate an IgE response leading to AGS, particularly after repeated tick bites. In addition, we highlight the value of passive tick surveillance programs, which led to public health reporting of this uncommon finding. Additional work will be needed to determine a possible link between I. pacificus or other Ixodes spp. ticks and AGS in the United States. Providers should consider AGS as a cause of anaphylaxis in the western United States. Public health practitioners across the United States should continue efforts focused on tick bite prevention, healthcare provider education, and improved tick and tickborne disease surveillance.
Dr. Butler is an allergist/immunologist in clinical practice with Kaiser Permanente in Seattle, Washington. His research interests focus on pediatrics, asthma and immunology, and diagnostic laboratory immunology.
Acknowledgments
We thank the patient for her cooperation and for allowing this case to be published and Alison Hinckley, Rebecca Eisen, and Paul Mead for their review and revision of this manuscript.
E.S. was partly supported by the Research Participation Program at the Centers for Disease Control and Prevention in Atlanta, which is administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the US Department of Energy and Centers for Disease Control and Prevention.
References
- Commins SP, Jerath MR, Cox K, Erickson LD, Platts-Mills T. Delayed anaphylaxis to alpha-gal, an oligosaccharide in mammalian meat. Allergol Int. 2016;65:16–20. DOIPubMedGoogle Scholar
- Van Nunen SA, O’Connor KS, Clarke LR, Boyle RX, Fernando SL. An association between tick bite reactions and red meat allergy in humans. Med J Aust. 2009;190:510–1. DOIPubMedGoogle Scholar
- Commins SP, James HR, Kelly LA, Pochan SL, Workman LJ, Perzanowski MS, et al. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-α-1,3-galactose. J Allergy Clin Immunol. 2011;127:1286–93.e6. DOIPubMedGoogle Scholar
- Young I, Prematunge C, Pussegoda K, Corrin T, Waddell L. Tick exposures and alpha-gal syndrome: A systematic review of the evidence. Ticks Tick Borne Dis. 2021;12:
101674 . DOIPubMedGoogle Scholar - Kersh GJ, Salzer J, Jones ES, Binder AM, Armstrong PA, Choudhary SK, et al. Tick bite as a risk factor for alpha-gal-specific immunoglobulin E antibodies and development of alpha-gal syndrome. Ann Allergy Asthma Immunol. 2023;130:472–8. DOIPubMedGoogle Scholar
- Thompson JM, Carpenter A, Kersh GJ, Wachs T, Commins SP, Salzer JS. Geographic distribution of suspected alpha-gal syndrome cases—United States, January 2017–December 2022. MMWR Morb Mortal Wkly Rep. 2023;72:815–20. DOIPubMedGoogle Scholar
- Crispell G, Commins SP, Archer-Hartman SA, Choudhary S, Dharmarajan G, Azadi P, et al. Discovery of alpha-gal–containing antigens in North American tick species believed to induce red meat allergy. Front Immunol. 2019;10:1056. DOIPubMedGoogle Scholar
- Commins SP. Diagnosis & management of alpha-gal syndrome: lessons from 2,500 patients. Expert Rev Clin Immunol. 2020;16:667–77. DOIPubMedGoogle Scholar
- British Columbia Centre for Disease Control. Ticks and tick-borne disease surveillance in British Columbia. June 2023 [cited 2024 Feb 22]. http://www.bccdc.ca/Documents/Ticks_and_Tick-Borne_Disease_Surveillance_BC.pdf
- Kwak M, Somerville C, van Nunen S. A novel Australian tick Ixodes (Endopalpiger) australiensis inducing mammalian meat allergy after tick bite. Asia Pac Allergy. 2018;8:
e31 . DOIPubMedGoogle Scholar - Hamsten C, Tran TAT, Starkhammar M, Brauner A, Commins SP, Platts-Mills TAE, et al. Red meat allergy in Sweden: association with tick sensitization and B-negative blood groups. J Allergy Clin Immunol. 2013;132:1431–4. DOIPubMedGoogle Scholar
- Fischer J, Riel S, Fehrenbacher B, Frank A, Schaller M, Biedermann T, et al. Spatial distribution of alpha-gal in Ixodes ricinus - A histological study. Ticks Tick Borne Dis. 2020;11:
101506 . DOIPubMedGoogle Scholar - Pisazka V, Duscher G, Hodžić A, Reider N, Allerberger F. Alpha-gal allergy after a tick bite in Austria. Wien Klin Wochenschr. 2019;131:385–8. DOIPubMedGoogle Scholar
- Cabezas-Cruz A, Hodžić A, Román-Carrasco P, Mateos-Hernández L, Duscher GG, Sinha DK, et al. Environmental and molecular drivers of the α-gal syndrome. Front Immunol. 2019;10:1210. DOIPubMedGoogle Scholar
- Binder AM, Commins SP, Altrich ML, Wachs T, Biggerstaff BJ, Beard CB, et al. Diagnostic testing for galactose-alpha-1,3-galactose, United States, 2010 to 2018. Ann Allergy Asthma Immunol. 2021;126:411–416.e1. DOIPubMedGoogle Scholar
Figure
Cite This ArticleOriginal Publication Date: March 19, 2025
1These authors contributed equally to this article.
Table of Contents – Volume 31, Number 4—April 2025
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Hanna Oltean, Washington State Department of Health, 1610 NE 150th St, Shoreline, WA 98155, USA
Top