Volume 5, Number 3—June 1999
Dispatch
Emergence of Related Nontoxigenic Corynebacterium diphtheriae Biotype mitis Strains in Western Europe
Abstract
We report on 17 isolates of Corynebacterium diphtheriae biotype mitis with related ribotypes from Switzerland, Germany, and France. Isolates came from skin and subcutaneous infections of injecting drug users, homeless persons, prisoners, and elderly orthopedic patients with joint prostheses or primary joint infections. Such isolates had only been observed in Switzerland.
Nontoxigenic Corynebacterium diphtheriae strains were recovered from approximately 1 per 1,000 throat swabs from immunized British military personnel in Germany from 1993 to 1995 (1). Such nontoxigenic C. diphtheriae biotype mitis isolates had been described in skin, throat, and blood cultures of Swiss injecting drug users; 32 of the isolates belonged to the same clone (2,3). Our study demonstrates that this clone and closely related clones occurred between 1990 and 1997 in two other European countries, and only sometimes in persons with poor hygiene.
Five C. diphtheriae isolates came from two laboratories in Zurich and Bern, Switzerland; 11 from four laboratories in Hamburg, Germany; and 1 from Paris, France (Table). They were identified in Zurich as C. diphtheriae biotype mitis (4); that is, they were nonlipophilic, were nitrate reductase-positive, and did not ferment glycogen. By polymerase chain reaction techniques (5), the diphtheria toxin gene was not detected in any isolate. For some isolates, the Elek test was also performed; it was consistently negative. For ribotyping, DNA was isolated, digested with either EcoRI or PvuII, electrophoresed, blotted, and probed for rDNA as described elsewhere (2,3). Disk susceptibility testing to tetracycline and MIC determinations were done according to National Committee for Clinical Laboratory Standards methods (6). All other bacteria isolated were also identified and serotyped in Zurich.
Many (10 [59%] of 17) C. diphtheriae isolates were from skin or subcutaneous infections (wounds, ulcers) in Swiss patients and were found with Staphylococcus aureus, Streptococcus pyogenes, group C/G streptococci, or (sometimes) with gram-negative rods. Most patients in this subgroup were injecting drug users, homeless persons, prisoners, and (with one exception) men with a mean age of 40 (30 to 58) years. Another subgroup (from Germany) consisted of six patients with joint or bone infections (mentioned briefly in an earlier publication on coryneform bacteria from such infections; a seventh patient had C. diphtheriae biotype gravis [7]). The six had been hospitalized at the Endo Clinic in Hamburg, which specializes in orthopedic surgery. Four had had implantations of hip endoprostheses and had been admitted for prosthetic infections (with coagulase-negative staphylococci or S. aureus); one pure culture of C. diphtheriae biotype mitis was obtained from a patient with a knee prosthesis, and one mixed culture with Peptostreptococcus magnus and Peptostreptococcus prevotii was obtained from a patient who had a purulent knee infection after a fracture. Their average age was 60 (23 to 82) years and, to our knowledge, none was a drug user. While the Hamburg isolates were recovered only once from every patient, their isolation dates stretched from March 1, 1995, until July 26, 1996. Three were isolated on the day of admission, and all of them were isolated by different technologists. Finally, one child (from France) had endocarditis with C. diphtheriae biotype mitis.
All isolates had identical antimicrobial susceptibility patterns. They were susceptible to amoxicillin (MIC, 0.25 µg/ml), amoxicillin-clavulanic acid (0.06 µg/ml), chloramphenicol (2 µg/ml), ciprofloxacin (0.25 µg/ml), clarithromycin (0.03 µg/ml), clindamycin (0.25 µg/ml), imipenem (0.03 µg/ml), penicillin (0.25 µg/ml), and vancomycin (1 µg/ml). In contrast, all strains were resistant to tetracycline in the disk diffusion test (inhibition zone diameter 10 mm to 11 mm); their MICs for tetracycline, doxycycline, and minocycline were 64 µg/ml, 16 µg/ml, and 16 µg/ml, respectively. While this type of isolated tetracycline resistance was typical for the isolates from Swiss injecting drug users (3), tetracycline resistance in nontoxigenic C. diphtheriae has otherwise very rarely been observed in Europe (8). It has, however, been reported in toxigenic C. diphtheriae isolates from Indonesia and, rarely, from Canada (9). The mechanism conferring this resistance in our strains has not been investigated.
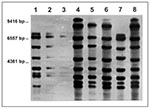
On analysis with restriction enzyme Pvu II, three different ribopatterns with eight bands each were found among the strains (Figure). These patterns, though distinct, shared five (A vs. C, B vs. C) and six (B vs. A) bands, respectively, and, therefore, may be considered related, as they would be if criteria for pulsed-field gel electrophoresis were applied (10). This view is supported by the fact that all strains had identical EcoRI patterns (not shown). All Swiss isolates were identical with the use of both enzymes, whereas both the Swiss pattern and a second pattern were found among the Hamburg isolates. The third pattern was found exclusively in the single French strain. The staphylococcal and streptococcal strains were not typed; they are no longer available to us.
Our isolates thus resemble those reported from Switzerland between 1990 and early 1996 (2,3), which were also often accompanied by S. aureus or beta-hemolytic streptococci. Such nontoxigenic C. diphtheriae mitis may cause endocarditis, arthritis, and osteomyelitis (11,12). Most of the 52 isolates from France (11), examined with restriction enzymes different from ours and not available to us, also belonged to one ribotype. Their relatedness to our strains is unknown; however, they were largely tetracycline-susceptible. The two throat isolates from St. Petersburg, Russia, associated with a fatal diphtherialike disease (12) were not typed or tested for antibiotic susceptibility.
The origin of the isolates we describe is unknown. They may have been present (but unrecognized) in the population for a long time. The mode of transmission is most likely common use of drug paraphernalia in the injecting drug use cases and in the endocarditis case; transmission is very difficult to explain in the orthopedic infection subgroup. Although the Swiss and the Danish borders are not close, migration and contacts are not uncommon among injecting drug users. The isolates may also have been distributed through the drugs themselves, as recently reported for S. pyogenes in Switzerland (13).
Our study may be representative for Switzerland and Germany, but since submitting C. diphtheriae strains to a central reference laboratory in these countries is not mandatory, we cannot estimate the frequency of these strains. These strains may also have spread to other European countries; this hypothesis can only be tested in a large multicenter study of European diphtheria reference laboratories.
Dr. Funke is director of clinical microbiology at Gärtner & Colleagues Laboratories in Weingarten, Germany. His areas of expertise are clinical and systematic bacteriology. Research interests include taxonomy and disease associations of coryneform bacteria and actinomycetes, as well as rapid methods for identification and susceptibility testing of medically relevant bacteria.
Acknowledgment
We thank J. Lüthy-Hottenstein and V. Pünter-Streit for excellent technical assistance and P. Das for her help in securing the strains from Hamburg. G. Funke is recipient of a European Society for Clinical Microbiology and Infectious Diseases research fellowship.
References
- Sloss JM, Hunjan RS. Incidence of non-toxigenic corynebacteria diphtheria in British military personnel in Germany. J Infect. 1996;33:139. DOIPubMedGoogle Scholar
- Gubler J, Huber-Schneider C, Gruner E, Altwegg M. An outbreak of non-toxigenic Corynebacterium diphtheriae infection: single bacterial clone causing invasive infection among Swiss drug users. Clin Infect Dis. 1998;27:1295–8. DOIPubMedGoogle Scholar
- Gruner E, Zuber PLF, Martinetti-Lucchini G, von Graevenitz A, Altwegg M. A cluster of non-toxigenic Corynebacterium diphtheriae infections among Swiss intravenous drug abusers. Med Microbiol Lett. 1992;1:160–7.
- Von Graevenitz A, Funke G. An identification scheme for rapidly and aerobically growing Gram-positive rods. Zentralbl Bakteriol. 1996;284:246–54.PubMedGoogle Scholar
- Martinetti-Lucchini G, Gruner E, Altwegg M. Rapid detection of diphtheria toxin by the polymerase chain reaction. Med Microbiol Lett. 1992;1:276–83.
- National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing; eighth informational supplement [NCCLS document M 100-S8]. Wayne (PA): The Committee; 1998.
- von Graevenitz A, Frommelt L, Pünter-Streit V, Funke G. Diversity of coryneforms found in infections following prosthetic joint insertion and open fractures. Infection. 1998;26:36–8. DOIPubMedGoogle Scholar
- Patey O, Bimet F, Emond JP, Estrangin E, Riegel P, Halioua B, Antibiotic susceptibilities of 38 non-toxigenic strains of Corynebacterium diphtheriae. J Antimicrob Chemother. 1995;36:1108–10. DOIPubMedGoogle Scholar
- Rockhill RC. Sumarmo, Hadiputranto H, Siregar SP, Muslihun B. Tetracycline resistance of Corynebacterium diphtheriae isolated from diphtheria patients in Jakarta, Indonesia. Antimicrob Agents Chemother. 1982;21:842–3.PubMedGoogle Scholar
- Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–9.PubMedGoogle Scholar
- Patey O, Bimet F, Riegel P, Halioua B, Emond JP, Estrangin E, Clinical and molecular study of Corynebacterium diphtheriae systemic infections in France. J Clin Microbiol. 1997;35:441–5.PubMedGoogle Scholar
- Rakhmanova AG, Lumio J, Groundstroem KWE, Taits BM, Zinserling VA, Kadyrova SN, Fatal respiratory tract diphtheria apparently caused by nontoxigenic strains of Corynebacterium diphtheriae. Eur J Clin Microbiol Infect Dis. 1997;16:816–20. DOIPubMedGoogle Scholar
- Streptokokken-Infektionen bei Drogensüchtigen in der Region Bern. Bulletin des Bundesamtes für Gesundheit. 1997;44:3.
Figure
Table
Cite This ArticleTable of Contents – Volume 5, Number 3—June 1999
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Alexander von Graevenitz, Department of Medical Microbiology, University of Zurich, Gloriastra sse 32, CH-8028 Zurich, Switzerland; fax: 411-634-4906
Top