Volume 7, Number 2—April 2001
THEME ISSUE
4th Decennial International Conference on Nosocomial and Healthcare-Associated Infections
State of the Art
Engineering Infection Control through Facility Design
Cite This Article
Citation for Media
Abstract
Many medical centers have modified their facility design to provide a safer environment for patients. From an infection control perspective, the primary objective of hospital design is to place the patient at no risk for infection while hospitalized. We describe historical landmarks about hospital design, modern facility design, and specific designs to prevent acquisition and spread of infections such as tuberculosis and aspergillosis.
While most hospitals are designed to control the spread of infection, this was not always the case. During the evolution of health care, most patients were cared for outside the hospital, and only the poor and disadvantaged received inpatient treatment. For most hospitals, care of the sick became difficult or unwanted. For example, when the statutes of the hospital of St. John, Bridgewater, were developed in 1219, Bishop Joscelin of Bath and Wells commented that "No lepers, lunatics, or persons having the falling sickness or other contagious disease, and no pregnant women or sucking infants, and no intolerable persons, even though they be poor and infirm, are to be admitted in the house; and if any such be admitted by mistake, they are to be expelled as soon as possible" (1). There are many similar cases of medieval English hospitals where admittance of sick persons was discouraged (2).
The delivery of babies in hospital is a relatively recent phenomenon: it evolved during the last half of the 20th century. Before then, maternity hospitals were not considered safe because of relatively high rates of death. It was not until the observations of Oliver Wendell Holmes and Ignaz Semmelweis that puerperal fever was thought to be a communicable disease transmitted from health-care workers to patients.
Semmelweis hypothesized that puerperal fever was spread by the hands of physicians and midwives. He noted that at the Vienna Lying-In Hospital the death rate was almost 10% for women who delivered in Division I, compared with 3% for women in Division II (3). Semmelweis' investigation determined that food, water, ventilation, or socioeconomic class did not account for these discrepancies. However, he observed that patients with prolonged labor were at increased risk and children born to infected mothers were also more likely to become ill. Conversely, women whose babies were born outside the hospital were less likely to develop fever. Semmelweis also noted that infection in Division I occurred sporadically and in clusters, whereas in Division II, no clustering occurred.
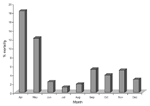
His analysis revealed that medical students, who were responsible for deliveries in Division I, often performed autopsies before assisting in deliveries, while midwives, who worked in Division II, did not. He theorized that disinfecting hands could prevent transmission of infection from a diseased cadaver to a pregnant patient (3). Therefore, on May 15, 1847, he required all medical students to wash their hands with chlorinated lime before assisting in deliveries, which resulted in a dramatic outcome (Figure).
Florence Nightingale made many observations about hospital design based on her experiences during the Crimean War. Her ideas regarding a sanitary environment meant rejecting the 18th-century concept of long hospital corridors. She commented that double wards were objectionable on every account primarily because they prevented nurses from being able to assess all their patients at the same time (4). She also observed that open windows interfered with the ventilation of hospital wards and allowed air from the wards to pass into the corridors. Nightingale believed that respiratory secretions were potentially dangerous, especially among the sick. Therefore, she said that depriving patients of appropriate ventilation "is nothing but manslaughter under the garb of benevolence" (5). Finally, she believed the sick should be isolated and that hospitals should be no more than two stories high. It was her contention that taller buildings interfered with sunlight and ventilation.
In 1875, after a large donation from Johns Hopkins, plans were developed to build a hospital in Baltimore, Maryland. Of five construction plans, two were substantially influenced by infection control. Norton Folsom, superintendent of Massachusetts General Hospital, believed that the hospital should be well ventilated and provide an isolation ward "for the occasional case so contagious or unpleasantly smelly that it cannot remain under the same roof with others" (6). A New York physician, Stephen Smith, believed that contagious patients should be separated from each other. In his plan, Smith classified patients into one of four categories: acutely contagious cases; uncomplicated infections and fever cases; acute medical and surgical cases; and completely noninfectious chronic disease cases. Further, he suggested that properly separating patients, with appropriate ventilation, was the most important facet of hospital planning.
In 1920, Asa Bacon of Chicago's Presbyterian Hospital noted that hospitals are hotels for sick people. One disgruntled patient commented to him following his discharge, "When I return, put me in a closet rather than in the ward!" (7). Bacon concluded that the most efficient hospital would contain all private rooms. His vision included a private toilet and lavatory in each room; a central kitchen and serving station; central linen supply instead of linen rooms on each floor; elimination of long corridors; dumbwaiters direct from central supply rooms; and pneumatic tubes to carry written requisitions. Bacon proposed these innovations 80 years ago, and today we take them for granted as integral to the modern medical center.
The Royal Infirmary in Aberdeen, Scotland, was specifically designed to prevent hospital-acquired infections in the surgical unit (8). Based on the recommendations of the Infirmary's Department of Bacteriology, no room had more than four beds, and 41% of the rooms were private or had only one bed. In addition, 10 private rooms surrounding central nurses' station were designed for "intensive nursing care." All rooms were mechanically ventilated, and 75% of the air was cleaned and then recirculated. The design also included an ISPIN (isolation, pre- and postoperative care including intensive nursing) unit with all private rooms placed between the operating room and the wards. This allowed "clean" surgeries to be separated from those with the potential of infection.
To minimize the risk for infection in hospitalized patients, infection control professionals should participate in facility design from a building's inception (9). This allows for identifying potential infection control issues early and provides an opportunity to design solutions prospectively. Infection control professionals also play an important role in educating architects, engineers, and construction workers about potential infection control risks and appropriate methods for reducing them. Because infection control professionals are often the only personnel with a clinical background working on the construction project, they need to visit the construction site frequently and completely understand the extent of the project. Because of the profound implications of inadequate oversight by infection control professionals, these expectations should be included in the hospital building contract (10). In addition, if the policies and procedures set forth by the infection control team are consistently ignored, the institution should fine the contractors.
As part of the planning process for constructing a new facility, an infection control risk assessment should be conducted to determine the potential risk for transmission of microorganisms within the hospital. In general, the risks can be classified as infections transmitted by air, water, or environment. The association between construction and the development of aspergillosis in immunocompromised patients has been known for decades (11), as has the association of hospital-acquired legionellosis and potable water (12). More recently, contamination of the hospital environment has been associated with transmission of Clostridium difficile (13), methicillin-resistant Staphylococcus aureus (MRSA) (14), and vancomycin-resistant enterococci (VRE) (15).
Aspergillus spp. are ubiquitous fungi, typically found in soil, decaying vegetation, and dust. Aspergillus spores are easily suspended in the air and survive for prolonged periods. Because of their size, they are easily inhaled, which can lead to invasive infection of both the upper and lower respiratory tracts in a susceptible host.
Epidemiologic evidence clearly correlates hospital acquisition of aspergillosis with Aspergillus spore counts (16). Therefore, installation of HEPA filters is essential in locations housing patients at high risk. While achieving a spore-free environment is an admirable goal, minimal concentrations of fungal spores in the environment are considered safe. In our new hospital, Northwestern Memorial, in Chicago, Illinois, the entire building is HEPA filtered because of the increasing number of immunosuppressed patients. Before opening the hospital, we performed air sampling to ensure the efficacy of the HEPA filter system and found that the composite fungal concentration and the Aspergillus spp. spore count were consistent with a highly filtered environment (Table).
Prevention of aspergillosis is particularly important for patients undergoing solid organ and bone marrow transplantation. In bone marrow transplant units, the air should be HEPA filtered with the air pressure in the room positive in relation to the corridor. In addition, rooms should be tightly sealed, especially around windows, and the air exchange rate should be high (>15 per hour) (17).
Proper health-care facility design can prevent hospital transmission of TB to patients and health-care workers. Ultimately, the interventions necessary to prevent hospital transmission of TB depend on the incidence of this disease in the community and have been published in detail (18). The Centers for Disease Control and Prevention recommends that patients requiring isolation for TB be placed in a room with negative airflow. These rooms should have frequent air exchanges (>12 per hour), and the air should be exhausted to the outside without recirculation. Doors to the rooms should be self-closing, and the walls, windows, ceiling, floor, and penetrations well sealed. These rooms should be monitored to ensure that they remain under negative pressure when occupied by a TB patient.
Infection control professionals play a substantial role in determining the appropriate location of negative-airflow rooms when a hospital is being designed. Ideally, they should be located in areas where patients at high risk will be cared for (e.g., emergency department, recovery room, bronchoscopy suite, ambulatory clinic, medical units).
Legionella is an important cause of community- and hospital-acquired lower respiratory tract infections. Person-to-person transmission of this organism has not been documented. Rather, infection is exclusively acquired from the environment, and hospital acquisition is well recognized (12,19,20). The most consistent observation about health-care acquired legionellosis is its association with potable water. The highest concentrations of the organism are found in hot-water storage tanks, cooling towers, and condensers.
Effective methods for disinfecting the hospital water supply include chlorination, thermal eradication, UV light, and metal ionization (16). At our new medical center, we elected to install a copper-silver ionization system. Despite the potential presence of Legionella in the water supply, routine culturing of water in the absence of proven or suspected hospital transmission is not recommended (21).
Hospital design should ensure that patients, especially immunocompromised patients, are at no greater risk for infection within the hospital than outside. Because the microbial flora of a health-care facility can be influenced by its design, infection control professionals play a major role in this aspect.
Bacteria on hospital floors predominantly consist of skin organisms, e.g., coagulase-negative staphylococci, Bacillus spp., and diptheroids (22); S. aureus and Clostridium spp. can also be cultured. However, infection risk from contaminated floors is small. Gram-negative bacteria are rarely found on dry floors, but may be present after cleaning or a spill. Nevertheless, these organisms tend to disappear as the surface dries (23).
The survival of microbes on carpeting, however, is different: they are present in larger numbers on this surface and they pose a greater risk for infection. Therefore, carpets should be vacuumed daily and periodically steam cleaned. Carpeting should be avoided in high-risk areas because the cleaning process may aerosolize fungal spores. Regardless of the flooring chosen, it should be easily cleanable and water resistant (9).
In general, pathogenic microorganisms do not readily adhere to walls or ceilings unless the surface becomes moist, sticky, or damaged (23). Little evidence exists that walls and ceilings are a major source for hospital infection. Wall coverings should be fluid resistant and easily cleaned, especially in areas where contact with blood or body fluids may occur (e.g., laboratories, operating rooms). Finishings around plumbing fixtures should be smooth and water resistant (9). In addition, pipe penetrations and joints should be tightly sealed. Acoustical tiles should be avoided in high-risk areas because they may support microbial growth when wet. False ceilings may harbor dust and pests that may contaminate the environment if disturbed, so should be avoided in high-risk areas unless adequately sealed. Ideally, walls and ceilings should have a smooth, impervious surface that is easy to clean with minimal likelihood of dust accumulation.
Infection control professionals are often consulted to recommend appropriate finishes and fixtures. The best finishes are durable and easy to clean. Surfaces that are porous or textured may be difficult to clean and might therefore harbor potentially pathogenic microbes (10). Furniture is thought to be a minor infection risk, but prolonged survival of VRE on chairs (24) and other environmental surfaces has been documented (25). MRSA and VRE have also been recovered from privacy curtains, scrub suits, and plastic aprons (26); whether contamination of these surfaces poses a risk to patients is unknown. However, survival of these pathogens for even a short time increases the possibility of their being acquired by patients or health-care workers and spread from one person to the next.
Handwashing is the single most important method to prevent hospital infections. Each patient room, examination room, and procedure room needs at least one sink (9). Optimally, it should be as close to the entrance of the room as possible and be large enough to prevent splashing. Too shallow a sink may cause contamination of hands by bacteria residing in the drain; this was linked to a hospital outbreak of multidrug-resistant gram-negative bacilli (27). Each sink should be equipped with a hands-free control, soap dispenser, and paper towel holder. Access to examination gloves and a trash receptacle should be readily available. We installed a dedicated sink at the entrance to every patient room to facilitate handwashing by health-care workers.
The design of health-care facilities has undergone substantial changes in large part because patients with impaired host defenses now represent an increasing proportion of hospitalizations. As a result, both design and renovation of these facilities present unique challenges and opportunities for infection control professionals, who are often the only clinical staff associated with construction projects. Early involvement in the process can make appropriate communication easier and protect patient safety. Ultimately, while time-consuming, participation in hospital design, construction, and renovation can serve as another marker of how infection control professionals improve the quality of patient care.
Dr. Noskin serves as medical director of the Infection Control and Prevention Department at Northwestern Memorial Hospital as well as their health-care epidemiologist. He is associate professor of medicine at Northwestern University Medical School and codirector of the Northwestern Memorial Hospital Infection Control and Prevention Project, a CDC-Sponsored Prevention Epicenter.
Dr. Peterson is director of the clinical microbiology division and principal investigator of the Northwestern Prevention Epicenter at Northwestern Memorial Hospital. His research focus is on molecular testing methods, antimicrobial agent resistance, and designing new strategies for managing reemerging bacteria.
Acknowledgments
The authors acknowledge the support of Northwestern Memorial Hospital, particularly Gary A. Mecklenburg, Kathleen G. Murray, and Lawrence L. Michaelis, for their generous support of the infection control program.
This work was supported in part by U.S. Public Health Service Grant UR8/CCU515081 and Northwestern Memorial Hospital.
References
- Maxwell-Lyte HC, ed. The Register of Thomas Bekynton, Bishop of Bath and Wells 1443-1465. Vol. 49. Somerset, UK: Somerset Record Society; 1934. p. 289.
- Carlin M. Medieval English hospitals. In: Granshaw L, Porter R, editors. The hospital in history. London: Routledge; 1989. p. 21-40.
- Semmelweis IF. The etiology, the concept and the prophylaxis of childbed fever. In: Pest CA, editor. Hartleben's Verlag-Expedition, 1861. [translated by Murphy FP; republished. Birmingham: Classics of Medicine Library; 1981].
- Nightingale F. Notes on hospitals. London: John W. Parker & Son; 1859. p. 11.
- Nightingale F. Notes on hospitals. London: John W. Parker & Son; 1859. p. 90-1.
- Chesney AM. The Johns Hopkins Hospital and the Johns Hopkins University School of Medicine. Baltimore: Johns Hopkins Press; 1943. p. 20-1.
- Bacon AS. Efficient hospitals. JAMA. 1920;74:123–6.
- Gainsborough H, Gainsborough J. Principles of hospital design. London: Architectural Press; 1964.
- American Institute of Architects. Guidelines for design and construction of hospital and health care facilities, 1996-97. Washington: American Institute of Architects Press; 1996.
- Carter CD, Barr BA. Infection control issues in construction and renovation. In: Herwaldt LA, Decker MD, editors. A practical handbook for hospital epidemiologists. Thorofare (NJ): Slack, Inc.; 1997:317-30.
- Kyriakides GK, Zinneman HH, Hall WH, Arora VK, Lifton J, DeWolf WC, Immunologic monitoring and aspergillosis in renal transplant patients. Am J Surg. 1976;131:246–52. DOIPubMedGoogle Scholar
- Doebbeling BN, Ishak MA, Wade BH, Pasquale MA, Gerszten RE, Groschel DH, Nosocomial Legionella micdadei pneumonia: 10 years experience and a case-control study. J Hosp Infect. 1989;13:289–28. DOIPubMedGoogle Scholar
- Kaatz GW, Gitlin SD, Schaberg DR, Wilson KH, Kauffman CA, Seo SM, Acquisition of Clostridium difficile from the hospital environment. Am J Epidemiol. 1988;127:1289–94.PubMedGoogle Scholar
- Rutala WA, Katz EBS, Sherertz RJ, Sarubbi FA Jr. Environmental study of a methicillin-resistant Staphylococcus aureus epidemic in a burn unit. J Clin Microbiol. 1983;18:683–8.PubMedGoogle Scholar
- Livornese LL, Dias S, Samel C, Romanowski B, Taylor S, May P, Hospital-acquired infection with vancomycin-resistant Enterococcus faecium transmitted by electronic thermometers. Ann Intern Med. 1992;117:112–6.PubMedGoogle Scholar
- Pannuti CS. Hospital environment for high-risk patients. In: Wenzel RP, editor. Prevention and control of nosocomial infections. Baltimore: Williams and Wilkins; 1997:463-89.
- Centers for Disease Control and Prevention. Guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. MMWR Morb Mortal Wkly Rep. 2000;49(RR-10):1–125.PubMedGoogle Scholar
- Centers for Disease Control and Prevention. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings. MMWR Morb Mortal Wkly Rep. 1994;43(RR-13):1–132.
- Helms CM, Massanari RM, Zeitler R, Streed S, Gilchrist MJ, Hall N, Legionnaires' disease associated with a hospital water system: a cluster of 24 nosocomial cases. Ann Intern Med. 1983;99:172–8.PubMedGoogle Scholar
- Neill MA, Gorman GW, Gilbert C, Roussel A, Hightower AN, McKinney RM, Nosocomial legionellosis, Paris, France; evidence for transmission by potable water. Am J Med. 1985;78:581–8. DOIPubMedGoogle Scholar
- Redd SC, Cohen ML. Legionella in the water: what should be done? JAMA. 1987;257:1221–2. DOIPubMedGoogle Scholar
- Ayliffe GAJ, Collins BJ, Lowbury EJL, Babb JR, Lilly HA. Ward floors and other surfaces as reservoirs of hospital infection. J Hyg (Camb). 1967;65:515–36. DOIPubMedGoogle Scholar
- Ayliffe GAJ, Babb JR, Taylor LJ. The hospital environment. In: Hospital-acquired infection: principles and prevention. Oxford: Butterworth-Heinemann; 1999. p. 109-21.
- Noskin GA, Bednarz P, Reiner S, Suriano T, Peterson LR. Persistent contamination of fabric covered furniture by vancomycin resistant enterococci: implications for upholstery selection in hospitals. Am J Infect Control. 2000;160:2819–22.PubMedGoogle Scholar
- Noskin GA, Stosor V, Cooper I, Peterson LR. Recovery of vancomycin resistant enterococci on fingertips and environmental surfaces. Infect Control Hosp Epidemiol. 1995;16:577–81. DOIPubMedGoogle Scholar
- Neely AC, Maley MP. Survival of enterococci and staphylococci on hospital fabrics and plastic. J Clin Microbiol. 2000;38:724–6.PubMedGoogle Scholar
- Gonzalez VR, Hougland PW, Vallejo KR, Price MF, Houston S, LaRocco M, An outbreak of Serratia marcescens in a cardiovascular intensive care unit: contaminated handwashing sinks as a reservoir. In: Program and abstracts of the 4th International Decennial Conference Nosocomial and Healthcare-Associated Infections; March 5-9, 2000; Atlanta. Atlanta: Centers for Disease Control and Prevention; 2000.
Figure
Table
Cite This ArticleTable of Contents – Volume 7, Number 2—April 2001
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Gary A. Noskin, Northwestern Memorial Hospital, 251 E. Huron Street, Feinberg 16-704, Chicago, IL 60611, USA: fax: 312-926-7845
Top