Volume 7, Number 5—October 2001
Research
Rapid Emergence of Ciprofloxacin-Resistant Enterobacteriaceae Containing Multiple Gentamicin Resistance-Associated Integrons, the Netherlands
Abstract
In a hematology unit in the Netherlands, the incidence of ciprofloxacin-resistant Enterobacter cloacae and Escherichia coli increased from <0.5% to 20.7% and <0.5% to 64%, respectively, from 1996 to 1999. Clonal spread of single genotypes of both ciprofloxacin-resistant E. coli and Enterobacter cloacae from patient to patient was documented by pulsed-field gel electrophoresis and random amplification of polymorphic DNA. In addition, genetically heterogeneous strains were isolated regularly. Integrons associated with gentamicin resistance were detected in Enterobacter cloacae and E. coli strains. Integron-containing E. coli were detected in all hematology wards. In contrast, in Enterobacter cloacae strains two integron types were encountered only in the isolates from one ward. Although in all patients identical antibiotic regimens were used for selective decontamination, we documented clear differences with respect to the nosocomial emergence of ciprofloxacin-resistant bacterial strains and gentamicin resistance-associated integrons.
Patients with severe neutropenia (neutrophil count <500/mL) are at risk for bacterial and fungal infections. In addition to exogenous routes of infection, the endogenous intestinal bacterial flora is a potential source of life-threatening bacteremia caused by gram-negative microorganisms. Patients are especially vulnerable who have prolonged periods of neutropenia (>10 days), a very low neutrophil count (<100/mL), and mucositis due to anticancer chemotherapy. To reduce the incidence of bacteremia, such patients often receive antibiotic prophylaxis, called selective decontamination of the gut. This prophylaxis is intended to eliminate potentially pathogenic bacterial species while maintaining native anaerobic flora. The fluoroquinolones (e.g., ciprofloxacin) appear to combine excellent activity, good bioavailability, and high concentrations in the gut, and thus provide an important component of the standard selective decontamination in many centers (1). Successful implementation of this method has been reported for liver transplant patients (2), patients with cirrhosis and acute upper gastrointestinal hemorrhage (3), and hematooncology patients (4,5). However, a drawback is that the use of broad-spectrum antibiotics may eventually lead to selection of antimicrobial resistance (6-8), especially when patients have received prior courses of antibiotic treatment (9). Concern about emergence of multidrug-resistant microorganisms is one of the main reasons that decontamination procedures have not been generally accepted.
Bacteria such as Escherichia coli and Enterobacter cloacae exist in the human gut, as well as the environment. These bacteria represent an important class of opportunistic pathogenic microorganisms. The number of strains that are resistant, especially to antibiotics that are frequently used for selective decontamination, is increasing. In the United States, Canada, Latin America, Sicily, Spain, and Kuwait, for example, ciprofloxacin is no longer the most effective drug for treating bloodstream infections caused by gram-negative bacilli (9-12). Recent French and Belgian studies presented detailed, nationwide analyses of the emergence of antibiotic-resistant strains of Enterobacter aerogenes and associated risk factors (13-15). The emergence of nosocomially disseminating clones can be explained by the fact that ciprofloxacin resistance is due to point mutations in DNA gyrase and topoisomerase genes. The latter genes are not known to be interchangeable or prone to recombination among resistant and susceptible enterobacterial isolates. Most bacterial strains derived from bloodstream infections acquired in northern European countries were still highly susceptible to ciprofloxacin (16,17). An additional, complicating factor is that antibiotic-resistance genes are frequently trapped in cassettes, the so-called integrons (18-20), which provide an efficient means for capturing and exchanging resistance genes. This implies that both bacterial strain characteristics and the exchange and dissemination of resistance genes need to be considered during ongoing outbreaks of infection (21).
Our molecular epidemiologic study was undertaken as part of an effort to understand the rapid spread of ciprofloxacin-resistant enterobacteriaceae in a hematooncology department. Subsequent microbiologic analysis of the E. coli and E. cloacae strains involved revealed a high prevalence of integron-associated gentamicin resistance as well. In addition to whole genome typing of the bacterial isolates, integron polymorphism was assessed with the help of a polymerase chain reaction restriction fragment-length polymorphism (PCR-RFLP) assay. The study environment comprised three clinical wards of one department of hematology, housed in two buildings: one at the south bank of Rotterdam harbor, containing two hematology wards where patients are treated for hematologic malignancies, and the other at the north bank, containing one similar ward. The prevalence of several antimicrobial resistance traits was assessed, isolates were characterized by pulsed-field gel electrophoresis (PFGE) or random amplification of polymorphic DNA (RAPD) analysis, and the presence and variability of integrons were determined.
Patients
All isolates were cultured from patients admitted to one of the three hematology wards (Table 1). Units A and B were located in the same building. Units B and C consisted of single-person rooms with anterooms and private bathrooms, negative air flow, and HEPA-filtered air supply. Gloves and gowns were worn by staff on all occasions. To prevent possible food-related infections, all foods were specifically selected: items likely to be contaminated with large numbers of bacteria (uncooked vegetable such as lettuce, fermented cheese, and several fruit species) were avoided. Unit A was made up of shared rooms only (up to four patients per room). All patients received the same selective decontamination procedure, which was started on the day of admission. The standard regimen involved ciprofloxacin (2 doses a day, either 500 mg orally or 400 mg intravenously). If ciprofloxacin-resistant gram-negative bacilli were present in surveillance cultures, patients received 4 doses a day of 200 mg colistin plus 5 mL of a 1 mg/mL colistin-containing solution, combined with 3 doses a day of 1 mL of 80 mg/mL tobramycin. Surveillance cultures were taken weekly from nose, throat, urine, vagina, and rectum.
Bacterial Strains and Ciprofloxacin Resistance
All strains belonging to the species E. cloacae or E. coli cultured in the microbiology laboratory of the Erasmus University Medical Center Rotterdam (EMCR), having an MIC for ciprofloxacin >2 mg/mL and isolated from November 1998 to October 1999, were selected from stock cultures. A general antimicrobial-resistance profile was generated by the commercial MicroScan Walk-A-Way system (Dade Behring, Paris, France) according to the manufacturer's instructions. On the basis of this analysis, all ciprofloxacin-resistant strains were identified and included in the study. The MICs of ciprofloxacin, ceftazidime, gentamicin, tobramycin, amikacin, cotrimoxazole, imipenem, and colistin were determined by agar dilution testing according to guidelines of the National Committee for Clinical Laboratory Standards (NCCLS) (22). Strains were classified as susceptible (S), intermediate (I), or resistant (R) according to NCCLS breakpoints. Finally, to define the nature of the gentamicin resistance, strains were phenotypically analyzed with the Vitek2 Advanced Expert System (bioMerieux, Lyon, France). Gram-negative susceptibility cards (ASTN-010) were inoculated with a 0.6 McFarland suspension of the strains involved. A description of the aminoglycoside resistance genes is provided. From November 1998 to October 1999, 164 isolates of ciprofloxacin-resistant enterobacteriaceae were retrieved from 45 patients. A total of 159 isolates were identified as either E. coli or E. cloacae; the five other isolates could not be identified unambiguously. Overall, 108 of 159 isolates from 33 patients (from 1 to 7 per patient) were available for further study. Fifty-one appeared to be E. coli and 57 belonged to the species E. cloacae. Of 33 patients, 6 showed clear evidence of clinically significant infection with E. coli (n = 5) or E. cloacae (n = 1). The other 27 patients were colonized with these resistant organisms, but had no clear symptoms or signs of clinical infection.
DNA Isolation
DNA isolation from individual colonies of cells was performed with the guanidinium-Celite protocol described by Boom et al. (23). DNA concentration was assessed by agarose gel electrophoresis of aliquots and staining by ethidium bromide in comparison with known amounts of lambda DNA. DNA was stored at a concentration of 5 ng/mL in 10 mM tris-HCl pH 8.0, 0.1 mM EDTA at -20°C.
Amplification and Characterization of Integrons
PCR mapping of integrons can be done on the basis of their 5' and 3' conserved regions. PCR was carried out in 100-mL volumes, essentially as described by Levesque et al. (24). The sequences for the 3' and 5' CS primers were GGCATCCAAGCAGCAAG and AAGCAGACTT-GACCTGA, respectively. Amplifications included 50 ng of bacterial DNA and cycling consisted of 35 repetitions of 1 min at 94°C, 1 min at 60°C, and 5 min at 74°C. Amplification products were analyzed by agarose gel electrophoresis. Besides length assessment, RFLP was determined for all amplicons. For these experiments, the endonuclease AluI was used according to the manufacturer's instructions (Boehringer-Mannheim, Mannheim, Germany).
Random Amplification of Polymorphic DNA (RAPD)
RAPD was performed for the E. coli strains essentially as described (25). Primers for amplification were ERIC-1 and ERIC-2 (26). The PCR protocol consisted of 40 cycles of denaturation (1 min at 94°C), annealing (1 min at 25°C) and chain extension (2 min at 74°C). Reaction products were analyzed by agarose gel electrophoresis; single differences in banding patterns among strains led to the definition of novel genotypes.
Pulsed-Field Gel Electrophoresis (PFGE)
Before PFGE, strains were grown overnight on Brucella blood agar plates (bioMerieux, Lyon, France). Cells were embedded in 0.5% low melting point InCert agarose (FMC Bioproducts, Landgraaf, the Netherlands) buffered in 5 mM tris-HCl pH 8.0, 50 mM Na2EDTA, 5 mM EGTA. The blocks were deproteinated by overnight incubation in the same buffer containing 1% sodium dodecyl sulfate and 1 mg/mL of proteinase K (Sigma Chemicals, St. Louis, MO) at 37°C. After extensive washing, blocks were stored at 4°C. Approximately 3x5-mm portions of the blocks were incubated in the presence of 40 units of the restriction endonuclease XbaI (Boehringer-Mannheim, Mannheim, Germany) in the appropriate buffer. Incubation at 37oC continued for 18 hours, after which the blocks were incorporated in 1% Seakem GTG agarose slabgels (FMC BioProducts, Rockland, ME). The restriction fragments were separated at a field strength of 6 V/cm for 20 hours at 14°C. The pulse time linearly increased from 5 sec to 35 sec during electrophoresis (27). Concatemers of lambda DNA were used as molecular size markers (BioRad, Veenendaal, the Netherlands). Gels were stained with ethidium bromide postelectrophoresis and photographed (Mitsubishi Copy Processor, Progress Control, Waalwijk, the Netherlands). DNA fingerprints were inspected visually. Single band differences were scored for the definition of separate genotypes, which is stricter than the guidelines of Tenover et al., which were not intended for outbreaks of infection or colonization monitoring (28).
Statistical Analysis
We used Fisher Exact or chi-square tests for analysis of the differences in incidence of fluoroquinolone resistance. A two-tailed test p-value of <0.05 was considered statistically significant.
Prevalence of Ciprofloxacin-Resistant Enterobacteriaceae
Our study was prompted by the apparent rise in the incidence of ciprofloxacin-resistant enterobacteriaceae in the hematology wards of the EMCR, as well as a fatal case of ciprofloxacin-resistant E. cloacae septicemia in a neutropenic patient with cancer. In the hospital as a whole, the incidence of ciprofloxacin resistance among isolates of E. coli and E. cloacae has been relatively stable, increasing slightly from 1996 to 1999 from 3.5% (65 of 1,879 isolates, one per patient) and 3.4% (12 [4.7%] of 358) to (111 of 2,367; p = 0.06) and 4.2% (27 of 549; p = 0.3) per species. Among patients in the hematology wards, however, the incidence of ciprofloxacin-resistant E. coli increased from <0.5% in 1996 to 20.7% (21 of 82; p = 0.0004) in 1999. Even more notable, the incidence of ciprofloxacin-resistant E. cloacae rose from <0.5% to 64% (16 of 25; p = 0.01) during the same period. Therefore, the small increase in the overall incidence of ciprofloxacin resistance among enterobacteriaceae in the entire hospital was in part attributable to the rise observed in the hematology department.
Overall Antimicrobial Resistance Patterns in Ciprofloxacin-Resistant Enterobacteriaceae
Resistance patterns were derived from MIC determinations in ciprofloxacin-resistant isolates from individual patients (Table 1). For patient 1, both a ciprofloxacin-resistant E. coli and an E. cloacae isolate were detected. Clear differences were observed in the proportions of E. coli or E. cloacae strains resistant to the various antibiotics tested (Table 2). Apparently, the E. cloacae strains are more often resistant toward ceftazidime, tobramycin, amikacin, cotrimoxazole, and, most notably, colistin. No strain was found to be resistant to imipenem.
Many of the enterobacters appeared multiresistant. The most prevalent types of resistance were CGTASL (17 [30%] of 56) and SL (16 [29%] of 56). The E. coli strains were more diverse with respect to their antibiograms, with the GTS- (12 [24%] of 50) and GS-types (9 [18%] of 50) the most prevalent multiresistant isolates. These figures are biased, since multiple isolates were included per patient, but also on an individual basis the SL type is the most prevalent of the enterobacter strains, with 7 of 18 patients colonized. For the E. coli strains, the S type is the most frequently encountered on a patient-to-patient basis (8 of 27). In most cases, serial isolates from individual patients shared the same antibiogram, but exceptions to this rule were observed.
In addition to the aminoglycoside MIC determinations, the aminoglycoside-modifying enzymes involved were defined by the Vitek2 Advanced Expert System (Table 1). The aminoglycoside resistance trait of the Enterobacter spp. is primarily determined by the putative presence of an AAC(6') gene (aminoglycoside resistance type E; Table 1). Incidentally, other combinations are documented. This type of resistance always correlated with the presence of an integron. For the E. coli strains, more heterogeneous aminoglycoside resistance gene contents were documented. Aminoglycoside resistance was never recorded in the absence of an integron.
Genetic Typing of the Enterobacteriaceae
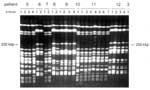
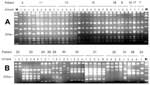
Genetic heterogeneity among the E. cloacae strains was assessed by PFGE (Figure 1). A relatively large proportion (approximately 33%) of the E. coli strains appeared to be reproducibly nontypeable by PFGE. During two independent experiments, no distinct banding patterns were generated, and only smears of degraded DNA were seen. Therefore, we decided to type all strains by using independent RAPD assays. With this approach, all strains appeared to be typeable. Moreover, the NotI PFGE data that were available for the PFGE-typeable subset of the strains were in full agreement with the RAPD data (results not shown). A survey of all PFGE- or RAPD-based genetic codes is presented in Table 1. E. cloacae strains were isolated from diverse clinical specimens derived from 18 patients. Sixteen PFGE types were determined. Among five patients who were colonized or infected by multiple types, two patients had three types and one patient was colonized by as many as four different PFGE genotypes. Several genotypes were encountered in more than one patient. Types B were isolated from three patients, and types E-J-M were detected in two patients each. These patients had not been clustered in time or space, so a common infectious source may exist that is unrelated to any of these patients. Type B occurred in 3 of 5 patients housed in Unit C, indicating that transmission is also a possibility in the highly restricted wards. Shared strains were also documented for patients in the high-level isolation, single-person units with anterooms and separate bathrooms, but could not be explained on the basis of patient-to-patient contacts. Most specific genotypes were confined to a single patient, where they were persistently found. The six patients who carried type C were admitted to ward A during overlapping time intervals (Figure 2), indicating that this type is an important nosocomial pathogen. The strains were derived from a variety of clinical specimens.
The isolates of E. coli were retrieved from 17 patients, and 15 different RAPD types were documented. Two patients were colonized with two strains, and two other patients with three different strains. One of these patients was transferred to another unit during his stay in the hematology department. Several RAPD type-strains were found in more than one patient. Types L and C were encountered twice, and type A was found in seven patients. For the type A strain, limited overlap in time and space was documented for the patients involved (late 1998 to early 1999). Four of them were in unit A (40% of all patients in that ward during the screening period were colonized with ciprofloxacin-resistant strains) (Figure 2). The possibility of a non-patient-associated environmental source remains, especially because bacterial dissemination was not restricted to the A unit, where physical isolation was less strict and several patients shared rooms. Putative explanations for spread are carriage by staff members or contamination of showers or toilets, but neither potential source was examined systematically during the study.
Integron Analysis
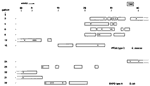
Detection of integron sequences was performed with a straightforward PCR assay. The results of these amplifications and the molecular sizes of the amplicons are summarized in Table 1. The integrons among the E. cloacae strains are clearly similar in structure and composition. In 26 (46%) of 56 strains, two different integrons were detected. In six additional strains, only the smaller one (2,000 bp) was detected. When the amplicons were digested with AluI, homogeneous RFLP patterns were observed, suggesting similarity of the two integrons (Figure 3A). The presence of the integrons correlates with resistance of the strains to gentamicin, tobramycin, and amikacin. Among the E. coli strains, greater diversity of PCR products was detected (Table 1) (Figure 3B). At least seven different-sized PCR products were visualized. Moreover, similar-sized integron amplicons can give rise to different RFLP patterns, indicating even more extensive heterogeneity. Thirty-five (70%) of 50 strains had one or more integrons. The association between integron presence and gentamicin resistance is still present, but is not as strong as for the E. cloacae strains.
We describe a study in a hematology department with patients housed in three clinical units in two buildings. As the antibiotic policy, especially the use of ciprofloxacin for selective decontamination, is identical throughout the department, the occurrence and means of spread of ciprofloxacin-resistant gram-negative bacilli can be measured against patient-related or environmental features. This study was initiated after we observed that the overall incidence of bacterial ciprofloxacin resistance in the hematology department had substantially exceeded that of the hospital as a whole. Moreover, these resistant organisms resulted in clinical infections in a significant proportion of the patients. Of the patients that we could track, 6 (18%) of 33 at any given time had bacteremia with a ciprofloxacin-resistant microorganism. Our study does not allow definite conclusions to be drawn on the relationship between the increased incidence of resistance on the one hand and bacterial genetic variability on the other. This would require analysis of susceptible strains as well, both from the hematology wards and other wards of the same hospital. However, our observations do provide insight into the molecular epidemiology of ciprofloxacin-resistant organisms and, separately, gentamicin resistance encoding integrons in the hematology department.
Outbreaks Caused by Ciprofloxacin-Resistant Microorganisms
PFGE has been used to study the dissemination of ciprofloxacin-susceptible Enterobacter strains in detail in various clinical settings (14,27,29-31). As such, the method is well accepted and has proven epidemiologic efficacy. We show dissemination of a PFGE type C E. cloacae strain in Unit A. This finding emphasized the need for adequate implementation of robust barriers in nursing hematology patients. Hand disinfection, both for patients and personnel, and the use of gloves and gowns by staff members are indispensable. After patients were specifically instructed on how to wash their hands after using the bathroom, the incidence of ciprofloxacin-resistant strains decreased (data not shown). However, in light of the overlapping genotypes, all clinical personnel involved should be aware of the potential for resistant bacteria to spread from patient to patient, within the environment, or among persons acting as potential vectors.
On two separate occasions, PFGE failed to type 33% of all ciprofloxacin-resistant E. coli strains. Therefore, we turned to random amplification of polymorphic DNA (RAPD) analysis, which has been used successfully to elucidate epidemic spread of E. coli (25,26) and appeared to be useful here as well. E. coli strains were 100% typeable with this latter technique. A possible nosocomial outbreak was assessed for RAPD type A. This type also occurred in the multiperson rooms in Unit A. The level of barriers (e.g., negative airflow, use of gloves and gowns) seems to be associated with the likelihood of an outbreak. A recent study performed in another Dutch university hospital identified 21 hematologic cancer patients colonized (79%) or infected (21%) with a ciprofloxacin-resistant E. coli strain over a 5-year period (32). An increase in incidence was noted over the years, whereas limited RAPD analyses suggested that nosocomial transmission had occurred. Overall, the observations from the two Dutch university hospitals suggest that the use of ciprofloxacin may predispose to outbreaks due to ciprofloxacin-resistant E. coli strains.
Integron Epidemiology
An integron is a genetic element that possesses a site at which cassettes of DNA can be integrated by site-specific recombination. The integron encodes an integrase enzyme that mediates the recombination events (18,19). Integrons are not independently mobile but may be found as part of transposons or plasmids, and the genes that they contain may not always be expressed with equal effectiveness (33). Such features may favor or limit the successful spread of integrons and may also provide a likely explanation for their ubiquity among gram-negative bacilli (34,35). A pan-European study recently revealed that >40% of all gram-negative clinical isolates harbor integrons and that the presence of integrons is associated with increased frequency of multiresistance and distinct resistance against aminoglycosides, quinolones, and beta-lactam compounds (36). In addition, the same authors suggested in a follow-up study that the conserved nature of the integrons that were identified could be an indication of the spread of entire integrons rather than the cassettes only (37). Integrons are assumed to play an important role in the dissemination of antimicrobial resistance (38). The contribution of integron cassettes to the prevalence of transferable aminoglycoside resistance has been demonstrated in a French hospital (39).
We have decribed the frequent occurrence of integron-encoded gentamicin resistance among nosocomial isolates of ciprofloxacin-resistant E. cloacae strains. Among the E. cloacae strains, two different integron types were encountered against a diverse background of chromosomes. This could be indicative of intraspecies dissemination of these particular elements, either as a whole or as the cassette content only, suggesting a strong species barrier. In case of the E. coli isolates, the integron types had greater diversity. Furthermore, the E. cloacae integron types were primarily confined to certain hematology units. Surprisingly, this suggests that a species barrier exists, prohibiting integron transfer between Enterobacter sp. and E. coli. In contrast to this observation, and substantiated by the fact that indiscriminate integrons occur in various bacterial strain types, exchange of integrons between strains of a single species seems to be effective. Furthermore, some integron types seem to be confined to a certain patient unit. In addition, our data indicate that in clinics where relatively large amounts of antibiotics are prescribed, resistance determinants may accumulate, especially in strains that show patient-to-patient transmission.
In conclusion, we describe differences in the nosocomial epidemiology of ciprofloxacin-resistant enterobacteriaceae. Besides variation in integron content, different species-specific integrons appear to circulate within the shared clinical environment. The observed differences between the spread of strains compared with antimicrobial resistance traits encoded by integrons merit additional investigations. We hypothesize that the use of various antibiotics, whether or not in combination, may have acted as a selective force in the emergence and dissemination of resistant microorganisms or their resistance-associated traits.
Dr. van Belkum is a molecular microbiologist working in the Department of Medical Microbiology and Infectious Diseases of the Erasmus University Medical Center in Rotterdam, the Netherlands. His research interests are in the field of molecular epidemiology of infectious agents and characterization of molecular determinants of host-microbe interactions during colonization and disease.
Acknowledgment
The authors thank the members of the group for infection control and hospital epidemiology, clinical personnel working in the department of hematology, and technicians from the department of medical microbiology and infectious diseases for their willing and conscientious collaboration.
References
- Klastersky J. Science and pragmatism in the treatment and prevention of neutropenic patients. J Antimicrob Chemother. 1998;41:13–24. DOIPubMedGoogle Scholar
- Emre S, Sebastian A, Chodoff L, Boccagni P, Meyers B, Sheiner PA, Selective decontamination of the digestive tract helps prevent bacterial infections in the early postoperative period after liver transplant. Mt Sinai J Med. 1999;66:310–3.PubMedGoogle Scholar
- Hsieh WJ, Lin HC, Hwang SJ, Hou MC, Lee FY, Chang FY, The effect of ciprofloxacin in the prevention of bacterial infection in patients with cirrhosis after upper gastro-intestinal bleeding. Am J Gastroenterol. 1998;93:962–6. DOIPubMedGoogle Scholar
- Carratalá J, Fernández-Sevilla A, Tubau F, Dominguez MA, Gudiol F. Emergence of fluoroquinolone resistant Escherichia coli in fecal flora of cancer patients receiving norfloxacin prophylaxis. Antimicrob Agents Chemother. 1996;40:503–5.PubMedGoogle Scholar
- Oethinger M, Conrad S, Kaifel K, Cometta A, Bille J, Klotz G, Molecular epidemiology of fluoroquinolone resistant Escherichia coli bloodstream isolates from patients admitted to European cancer centers. Antimicrob Agents Chemother. 1996;40:387–92.PubMedGoogle Scholar
- Carratalá J, Fernández-Sevilla A, Tubau F, Callis M, Gudiol F. Emergence of quinolone-resistant Escherichia coli bacteremia in neutropenic patients with cancer who have received prophylactic norfloxacin. Clin Infect Dis. 1995;20:557–60. DOIPubMedGoogle Scholar
- Cruciani M, Rampazzo R, Malena M, Fazzarini L, Todeschini G, Messori A, Prophylaxis with fluoroquinolones for bacterial infections in neutropenic patients: a meta-analysis. Clin Infect Dis. 1996;23:795–805. DOIPubMedGoogle Scholar
- Lingnau W, Berger J, Javorsky F, Fille M, Allerberger F, Benzer H. Changing bacterial ecology during a five-year period of selective intestinal decontamination. J Hosp Infect. 1998;39:195–206. DOIPubMedGoogle Scholar
- Gairau J, Xercavins M, Rodriguez-Carballeira M, Gomez-Vera J-R, Coll I, Vidal D, Emergence and dissemination of quinolone-resistant Escherichia coli in the community. Antimicrob Agents Chemother. 1999;43:2736–41.PubMedGoogle Scholar
- Blandino G, Caccamo F, di Marco R, Speciale A, Nicoletti G. Epidemiology of antibiotic resistance in human isolates of enterobacteriaceae in Sicily. J Chemother. 1990;2:40–4.PubMedGoogle Scholar
- Diekema DJ, Pfaller MA, Jones RN, Doern GV, Winokur PL, Gales AC, Survey of bloodstream infections due to gram-negative bacilli: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada and Latin America for the SENTRY antimicrobial surveillance program, 1997. Clin Infect Dis. 1999;29:595–607. DOIPubMedGoogle Scholar
- Jamal WY, El-Din K, Rotimi VO, Chugh TD. An analysis of hospital-acquired bacteraemia in intensive care unit patients in a university hospital in Kuwait. J Hosp Infect. 1999;43:49–56. DOIPubMedGoogle Scholar
- Bornet C, Davin-Regli A, Bosi C, Pages JM, Bollet C. Imipenem resistance of Enterobacter aerogenes mediated by outer membrane permeability. J Clin Microbiol. 2000;38:1048–52.PubMedGoogle Scholar
- Bosi C, Davin-Regli A, Bornet C, Mallea M, Pages JM, Bollet C. Most Enterobacter aerogenes strains in France belong to a prevalent clone. J Clin Microbiol. 1999;37:2165–9.PubMedGoogle Scholar
- Ronveaux O, de Gheldre Y, Glupczynski Y, Struelens M, de Mol P. Emergence of Enterobacter aerogenes as a major antibiotic-resistant nosocomial pathogen in Belgian hospitals. Clin Microbiol Infect. 1999;5:622–7. DOIPubMedGoogle Scholar
- Digranes A, Solberg CO, Sjursen H, Skovlund E, Sander J. Antibiotic susceptibility of blood culture isolates of enterobacteriaceae from six Norwegian hospitals, 1991-1992. APMIS. 1997;105:854–60. DOIPubMedGoogle Scholar
- Osterblad M, Pensala O, Peterzens M, Heleniusc H, Huovinen P. Antimicrobial susceptibility of enterobacteriaceae isolated from vegetables. J Antimicrob Chemother. 1999;43:503–9. DOIPubMedGoogle Scholar
- Bennett PM. Integrons and gene cassettes: a genetic construction kit for bacteria. J Antimicrob Chemother. 1999;43:1–4. DOIPubMedGoogle Scholar
- Fluit AC, Schmitz FJ. Class I integrons, gene cassettes, mobility and epidemiology. Eur J Clin Microbiol Infect Dis. 1999;18:761–70. DOIPubMedGoogle Scholar
- Mazel D, Davies J. Antibiotic resistance in microbes. Cell Mol Life Sci. 1999;56:742–54. DOIPubMedGoogle Scholar
- Chiew YF, Yeo SF, Hall LM, Livermore DM. Can susceptibility to an antimicrobial be restored by halting its use? The case of streptomycin versus enterobacteriaceae. J Antimicrob Chemother. 1998;41:247–51. DOIPubMedGoogle Scholar
- National Committee for Clinical Laboratory Standards. Performance guidelines for antimicrobial susceptibility testing: Fifth Informational Supplement M100-5. Villanova (PA): American Society for Microbiology; 1994.
- Boom R, Sol CJA, Salimans MMM, Jansen CL, Wertheim van Dillen PME, van der Noorda J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990;28:495–503.PubMedGoogle Scholar
- Levesque C, Piche L, Larose C, Roy PH. PCR mapping of integrons reveals several novel combinations of resistance genes. Antimicrob Agents Chemother. 1995;39:185–91.PubMedGoogle Scholar
- Van Belkum A, van Leeuwen W, Kluytmans J, Verbrugh HA. Molecular nosocomial epidemiology: high speed typing of microbial pathogens by arbitrary primed polymerase chain reaction assays. Infect Control Hosp Epidemiol. 1995;16:658–66. DOIPubMedGoogle Scholar
- Versalovic J, Koeuth T, Lupski JR. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991;19:6823–31. DOIPubMedGoogle Scholar
- Shi ZY, Liu PYF, Lau YJ, Lin YH, Hu BS. Epidemiological typing of isolates from an outbreak of infections with multi-drug resistant Enterobacter cloacae by repetitive extragenic palindromic unit b1-primed PCR and pulsed field gel electrophoresis. J Clin Microbiol. 1996;34:2784–90.PubMedGoogle Scholar
- Tenover FC, Arbeit RD, Goehring RV, Mickelsen PA, Murray BE, Persing DH, Interpreting chromosomal DNA restriction patterns produced by pulsed field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–9.PubMedGoogle Scholar
- De Gheldre Y, Maes N, Rost F, de Ryck R, Clevenbergh P, Vincent JL, Molecular epidemiology of an outbreak of multidrug-resistant Enterobacter aerogenes infections and in vivo emergence of imipenem resistance. J Clin Microbiol. 1997;35:152–60.PubMedGoogle Scholar
- Haertl R, Bandlow G. Epidemiological fingerprinting of Enterobacter cloacae by small-fragment restriction endonuclease analysis and pulsed field gel electrophoresis of genomic restriction fragments. J Clin Microbiol. 1993;31:128–33.PubMedGoogle Scholar
- Van Nierop WH, Duse AG, Stewart RG, Bilgeri YR, Koornhof HJ. Molecular epidemiology of an outbreak of Enterobacter cloacae in the neonatal intensive care unit of a provincial hospital in Gauteng, South Africa. J Clin Microbiol. 1998;36:3085–7.PubMedGoogle Scholar
- Van Kraaij MGJ, Dekker AW, Peters E, Fluit A, Verdonk LF, Rozenberg-Arska M. Emergence and infectious complications of ciprofloxacin resistant Escherichia coli in haematological cancer patients. Eur J Clin Microbiol Infect Dis. 1998;17:591–9. DOIPubMedGoogle Scholar
- Collis CM, Hall RM. Expression of antibiotic resistance genes in the integrated cassettes of integrons. Antimicrob Agents Chemother. 1995;39:155–62.PubMedGoogle Scholar
- Brown HJ, Stokes HW, Hall RM. The integrons In0, In2 and In5 are defective transposon derivatives. J Bacteriol. 1996;178:4429–37.PubMedGoogle Scholar
- Kholodii GY, Mindlin SZ, Bass IA, Yurieva OV, Minakhina SV, Nikiforov VG. Four genes, two ends and a res region are involved in transposition of Tn5053: a paradigm for a novel family of transposons carrying either a mer operon or an integron. Mol Microbiol. 1995;17:1189–200. DOIPubMedGoogle Scholar
- Martinez-Freijo P, Fluit AC, Schmitz FJ, Greks VS, Verhoef J, Jones ME. Class I integrons in gram-negative isolates from different European hospitals and association with decreased susceptibility to multiple antibiotic compounds. J Antimicrob Chemother. 1998;42:689–96. DOIPubMedGoogle Scholar
- Martinez-Freijo P, Fluit AC, Schmitz FJ, Verhoef J, Jones ME. Many class I integrons comprise distinct stable structures occurring in different species of enterobacteriaceae isolated from widespread geographic regions in Europe. Antimicrob Agents Chemother. 1999;43:686–9.PubMedGoogle Scholar
- Sundstrom L. The potential of integrons and connected programmed rearrangements for mediating horizontal gene transfer. APMIS. 1998;84:37–42. DOIPubMedGoogle Scholar
- Sallen B, Rajoharison A, Desvarenne S, Mabilat C. Molecular epidemiology of integron-associated antibiotic resistance genes in clinical isolates of enterobacteriaceae. Microb Drug Resist. 1995;1:195–202. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 7, Number 5—October 2001
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Alex van Belkum, Erasmus University Medical Center Rotterdam EMCR, Department of Medical Microbiology and Infectious Diseases, Dr. Molewaterplein 40, 3015 GD Rotterdam, the Netherlands; fax: 00-31-10-4633875
Top