Volume 7, Number 5—October 2001
Dispatch
First Epidemic of Echovirus 16 Meningitis in Cuba
Abstract
From April to September 2000, an epidemic of aseptic meningitis spread throughout Cuba, with 16,943 reported cases. Virologic studies identified echovirus 16 as the cause of this epidemic. This is the first reported isolate of echovirus 16 from patients with viral meningitis in Cuba.
From April 30 to September 2000, Cuban health authorities reported a marked increase in acute aseptic meningitis cases. The peak incidence occurred in July (49.6 cases per 100,000 population) (Figure 1). The index cases appeared in Cienfuegos Province, in the central part of the country. Subsequently, the disease was widely distributed: 16,943 cases were reported from April to September 2000.
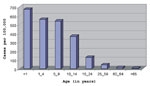
Most patients were children >15 years old. The age-specific peak incidence occurred in infants >1 year of age, but none were neonates (Figure 2). Vomiting (91.5%), headache (88.1%), and fever (72.8%) were the predominant clinical manifestations; few patients had diarrhea (11.8%) or skin rash (6.8%). Cerebrospinal fluid (CSF) cell counts showed >100 leukocytes/mm3 in 40% of patients. The rest of the CSF counts were 10-100 leukocytes/mm3. No deaths related to aseptic meningitis were reported, and all patients recovered completely.
To establish the outbreak-associated enterovirus, 54 CSF, 76 fecal samples, and 31 paired sera from acute and convalescent phases were obtained from 98 children with symptoms suggestive of aseptic meningitis. CSF and fecal samples were collected only once per child, at onset of symptoms. The children were admitted into hospitals in 11 of Cuba's 14 provinces. Specimens were collected from May 5 to August 11, 2000, and transported frozen to our laboratory.
We used conventional methods for diagnosis of enterovirus and an in-house-developed reverse transcriptase-nested polymerase chain reaction (RT-N-PCR) assay of CSF specimens. For the enterovirus genome detection, RNA was extracted from 250 µL of CSF using TRIzol (Life Technologies TM, GIBCO BRL; Grand Island, NY), according to the manufacturer's instructions. RNA amplification was performed by the method of Kilpatrick et al. (1), except that two amplification rounds were used. Oligonucleotides were derived from the 5'noncoding region (5'NCR), a highly conserved zone in enterovirus serotypes that allows a near-universal amplification of the enteroviruses (2,3).
Specificity of the RT-N-PCR assay was confirmed by detection of amplification products of RNA extracted from cell culture fluids infected with polioviruses sabin 1-3, coxsackievirus A9 and A16, and the most common epidemic types of echoviruses (echovirus 4,6,9,11,30), as well as by the absence of amplified cDNA with RNA extracted from herpesvirus family-infected and noninfected monkey kidney cells (Vero). The recognized sensitivity (0.01 50% tissue culture infective dose) and specificity of our enterovirus-RNA detection assay allowed us to detect specific enterovirus RNA sequences in 46.3% (25 of 54) of CSF specimens. We do not believe that positive results were due to PCR contamination because universal precautions were adopted (4). An equal number of test samples and reagent controls were processed in each test batch to prevent contamination of RNA extraction by extraneous nucleic acid sequences; PCR reagents were amplified by PCR. The reagent controls' reaction verified the absence of contamination at all stages of the PCR process.
The application of this in-house RT-N-PCR assay guaranteed the rapid etiologic diagnosis of this epidemic. However, our RT-N-PCR is unable to determine the enterovirus serotype, which is necessary to understand the epidemiology of enterovirus infections.
For enterovirus isolation, 200 µL of CSF and fecal specimens were inoculated in duplicate into tubes covered with monolayers of fibroblastic diploid embryonic human cells (PhuE-1) and Vero cells. From the 76 fecal specimens inoculated, 45 (59.2%) induced cytopathic effect (CPE). This CPE was only evident in the PhuE-1 cell monolayers; Vero cells remained unchanged. Viral isolation was possible from all the tubes showing CPE. However, no enterovirus strains were recovered by cell culture isolation from any CSF specimen. These discrepancies could be explained by the massive excretion of viral particles in feces. Moreover, previous studies have demonstrated that enteroviruses are isolated from CSF in only a few cases with acute aseptic meningitis because viral particles are present in low titers in CSF (5-7). Nevertheless, a higher positivity was obtained from 20 CSF samples when RT-N-PCR was used than for 20 samples of feces from the same patients when evaluated by viral isolation. Only 10 of the 16 CSF specimens positive by PCR could be correlated with a positive fecal culture, whereas the 4 PCR-negative CSF samples correlated well with the absence of viral isolation from the related fecal specimens.
CPEs produced by isolated strains were typical of those characterizing enteroviruses (e.g., cell rounding followed by shrinkage and degeneration of the cell sheet). However, at the beginning of the epidemic, isolated strains produced a CPE that progressed slower (slow-CPE) than typical enterovirus isolates do. The inoculated cultures had to be subpassaged weekly at least 5 times to obtain the typical degenerative CPE of enterovirus. By the late stages of the epidemic, CPE produced by isolated strains became evident at the first passage (fast-CPE). No slow-CPE isolates were detected late in the epidemic, and there was no evidence of "fast CPE-to-slow CPE" reversion of isolates late in the epidemic. Nevertheless, no changes in the clinical outcome of the infection were observed during the epidemic. It is tempting to speculate that the genetic constitution of the selected viral population could be substantially different from that of the original strain. This phenomenon could explain the variation in CPE during the epidemic.
All strains from the epidemic were identified as echovirus 16 by a neutralization test using Lim-Benyesh-Melnick antisera pools. To corroborate the infecting serotype, presence of a fourfold or greater increase of type-specific virus-neutralizing antibody titers between acute- and convalescent-phase serum specimens was determined; 54.8% (17 of 31) of the patients' sera exhibited a significant rise of neutralizing antibody titer against the isolated strains. The geometric mean titers of the first and second sera were 1:3.4; and 1:22.4, respectively.
Previous studies in Cuba have estimated the circulation of enterovirus in patients with meningitis. From 1972 to 1999, seven meningitis outbreaks caused by an enterovirus occurred: echovirus 4 (1972 and 1985-86), coxsackievirus B5 (1976 and 1995), coxsackievirus A9 (1990-1991), echovirus 30 (1994), and echovirus 9 (1999). Other enteroviruses (coxsackievirus and numerous echoviruses) were identified from sporadic cases of viral meningities during nonepidemic periods (6). Before this epidemic, aseptic meningitis caused by echovirus 16 had never been recognized in Cuba.
Nevertheless, the age distribution in this outbreak suggests previous exposure and immunity to echovirus 16 in older persons (Figure 2). The age group distribution was also similar to that in previously described outbreaks of aseptic meningitis in Cuba (6). A possible explanation for this age distribution is that an antigenically related virus without potential to cause acute aseptic meningitis circulated in the population before year 2000 and induced an immune response. Alternatively, maternal antibodies against this virus may protect neonates from infection.
Factors influencing the prevalence of enteroviruses are poorly understood. The ease with which a type of nonpolio enterovirus can be isolated, however, is likely to be a major determinant of the frequency with which it is reported (5,8). Echovirus 16 isolates associated with sporadic cases of aseptic meningitis have probably been infrequently reported in previous years because of the difficulties in tissue culture propagation.
According to data collected through the U.S. National Enterovirus Surveillance System, echovirus 16 is routinely isolated, but the frequency of its isolation is low in most years (i.e., 3 to 5 cases per year) (Centers for Disease Control and Prevention, unpub. data).
To our knowledge, the last Cuban outbreak of aseptic meningitis associated with echovirus 16 infections occurred in Tajimi City, Gifu prefecture, in 1984. Diverse etiologic agents (coxsackievirus B1, B4, and B5) were also reported during the outbreak (9).
The emergence of echovirus 16 associated with a very large-scale meningitis epidemic in Cuba should alert public health officials to the potential for epidemics associated with this serotype in other areas of the world.
The overall genetic diversity and molecular evolution in echovirus 16 strains and the correlation with the epidemiologic features of echovirus 16-associated disease have not yet been studied. The availability of the viral isolates, together with the massive clinical and epidemiologic data from this epidemic, represents an unprecedented opportunity to study the emergence of echovirus 16 strains and their subsequent molecular evolution.
Lic. Sarmiento is a virologist at the "Pedro Kouri" Tropical Medicine Institute. His research interests include enteroviral disease outbreak investigations and pathogenesis of enteroviral diseases.
Acknowledgment
The authors thank Carlos Suarez for critical review of the manuscript and Mark Pallansch and Silvia Peñaranda for helpful comments.
References
- Kilpatrick DR, Nottay B, Yang CF, Yang SJ, Da Silva E, Pallansch M, Serotype-specific identification of polioviruses by PCR using primers containing mixed-base or deoxyinosine residues at position of codon degeneracy. J Clin Microbiol. 1998;36:352–7.PubMedGoogle Scholar
- Zoll GJ, Melchers WJ, Kopecka H, Jambroes G, van der Poel HJ, Galama JM. General primer-mediated polymerase chain reaction for detection of enteroviruses: Application for diagnostic routine and persistent infections. J Clin Microbiol. 1992;30:160–5.PubMedGoogle Scholar
- Yang CF, De L, Yang SJ, Gómez JR, Cruz JR, Halloway BP, Genotype-specific in vitro amplification of sequences of the wild type 3 polioviruses from México and Guatemala. Virus Res. 1992;24:277–96. DOIPubMedGoogle Scholar
- Kwok S, Higuchi R. Avoiding false positives with PCR. Nature. 1989;339:237–8. DOIPubMedGoogle Scholar
- Morens DM, Pallansch MA. Epidemiology. In: Rotbart HA, editor. Human enterovirus infections. Washington: American Society for Microbiology Press; 1995. p.3-23.
- Mas P, Comellas M, Marrero M, Jacobo M, Palomera R. Meningoencefalitis por enterovirus en Cuba. Estudio de 14 años. Rev Cubana Pediatr. 1992;64:16–21.
- Anderoletti L, Damman NB, Dewilde A, Vallee L, Cremer R, Hober D, Comparison of use of cerebrospinal fluid, serum, and throat swab specimens in diagnosis of enteroviral acute neurological infection by a rapid RNA detection PCR assay. J Clin Microbiol. 1998;36:589–91.PubMedGoogle Scholar
- Strikas R, Anderson L, Parker R. Temporal and geographic patterns of isolates of nonpolio enterovirus in the United States, 1970-1983. J Infect Dis. 1986;153:346–51. DOIPubMedGoogle Scholar
- Miwa C, Watanabe Y. Diversity of etiological agent associated with aseptic meningitis-a survey on an epidemic in Tajimi City, Gifu Prefecture in 1984. Kansenshogaku Zasshi. 1990;64:794–801.PubMedGoogle Scholar
Figures
Cite This ArticleTable of Contents – Volume 7, Number 5—October 2001
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Luis Sarmiento Perez, Department of Virology, "Pedro Kouri" Tropical Medicine Institute, Autopista Novia del Mediodía, km 6, PO Box 601, Marianao 13, Havana, Cuba; fax: 53-7-220633 and 53-7-246051
Top