Volume 9, Number 3—March 2003
Dispatch
Mycobacterium celatum Pulmonary Infection in the Immunocompetent: Case Report and Review
Abstract
Mycobacterium celatum has been shown to cause disease in immunocompromised patients. We report a case of serious pulmonary infection caused by M. celatum in an apparently immunocompetent patient and review the characteristics of two other reported cases. Clinical and radiologic symptoms and signs included cough, malaise, and weight loss associated with cavitary lesions and pulmonary infiltrates. Although M. celatum is easy to detect in clinical specimens by liquid and solid media, it may be misidentified as a member of the M. tuberculosis complex or as M. xenopi. M. celatum pulmonary infection appears to respond to antimycobacterial chemotherapy, particularly with clarithromycin.
Mycobacterium celatum was first described in 1993 as a new species whose mycolic acid pattern closely resembled that of M. xenopi (xenopi-like) but was biochemically indistinguishable from M. avium complex (MAC) (1). By sequencing studies of the 16S rRNA gene, now known to exist in two different copies within M. celatum genome (2), three different types could be differentiated (1,3). Cross-reactivity with the Accuprobe assay (Gen-Probe Inc., San Diego, CA) for the M. tuberculosis complex (MTB) was observed by probing types 1 and 3 but not type 2 (3,4). DNA sequencing showed that M. celatum type 1 differs by one nucleotide from the probe used in the Accuprobe assay, type 2 differs by four nucleotides (5), and the type 3 DNA sequence for the probe region is unknown.
Although M. celatum has been reported to cause infection mainly in AIDS patients (6–10), recently a growing amount of clinical evidence indicates that the infection can lead to serious disease in immunocompetent subjects as well (11–13). In this case, the principles for diagnosis of diseases caused by nontuberculous mycobacteria, recently updated by the American Thoracic Society (14), need to be properly followed. We report a well-documented case of a pulmonary disease with this mycobacterium in an apparently immunocompetent woman and review the characteristics of two similar published cases.
A 63-year-old, HIV-negative woman was referred in June 2000 by a general practitioner to the outpatient pulmonary service because she had exhibited a fever and night sweats for 3 months. The patient’s medical history included a mild hypertensive cardiovascular disease and a pulmonary tuberculosis episode when she was 16 years old. Her father and brother had pulmonary tuberculosis. She lived in an urban area and had no history of acoholism, smoking, or use of steroids or immunosuppressive drugs. No other pulmonary diseases that could potentially lead to bronchiectasis and further mycobacterial colonization were reported. Physical examination revealed no pathologic findings except a strong positive reaction (24 mm of induration) with tuberculin skin test (5 U of purified protein derivative–standard [PPD-S]). Results of clinical laboratory tests were unremarkable, apart from an elevated erythrocyte sedimentation rate (77 mm/h). A computed tomographic (CT) scan of the chest showed multifocal bronchiectases in the middle right lobe, multiple small nodules in the lower right lobe, and a cavitary lesion in the upper right lobe, which appeared to have thin walls and was surrounded by a scant or absent inflammatory reaction. Bronchoscopy showed no inflammatory mucosal changes on the right side. Smears of bronchial washings were negative for acid-fast bacilli (AFB), and smears of two sputum specimens were positive, while all specimens gave negative results when tested by a ligase chain reaction commercial assay specific for MTB. A reactivation of tuberculosis was assumed, and chemotherapy with isoniazid, rifampin, and ethambutol was begun. Cultures of bronchial washing and sputum specimens produced a slow-growing Mycobacterium, which was identified as M. celatum in July 2000. The isolate was not considered clinically important, and chemotherapy was unchanged.
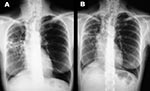
In December 2000, although the general condition of the patient had slightly improved after 6 months of treatment, a control CT scan showed a worsening (enlargement) of the nodular lesions. Smears of two sputum specimens and one bronchial washing specimen were positive for AFB, whereas all specimens were negative when tested by the MTB ligase chain reaction assay. All of these specimens yielded M. celatum. On the basis of susceptibility data obtained from the first M. celatum isolate, chemotherapy was changed to a schedule that included isoniazid, ethambutol, and clarithromycin, which lasted until November 2001. This regimen proved to be well tolerated by the patient. The bacteriologic results (specimens from two additional bronchial washings were negative for AFB by smear and culture) and clinical response were good. In December 2001, chest x-ray examinations and a CT scan showed a considerable reduction of nodular lesions as well as improvement of lung cavitation (Figure). The patient was considered cured after taking medication for approximately 18 months.
Clinical, microbiologic, and radiologic data as well, as information on response to treatment and follow-up, were obtained from the patient’s records. All material was carefully reviewed to evaluate the clinical significance of the findings according to the criteria proposed by the American Thoracic Society (14).
We examined sputum and bronchoaspirate specimens for acid-fast organisms. They were stained with routine Ziehl-Neelsen stain and cultured by standard procedures (15) by using a combination of radiometric Bactec system (Becton Dickinson Biosciences, Sparks, MD) and Löwenstein-Jensen medium. Amplification for MTB was performed by the LCx-MTB assay (Abbott, Diagnostics Division, Chicago, IL). Conventional identification and growth tests were performed according to standard methods (15). We tested recovered strains with the Accuprobe system using specific probes for MAC and MTB. Assays were run from liquid culture setting the unbound probe hydrolysis incubation time at 5 and 10 min and temperature at 60° ± 1°C (16). The amount of chemiluminescence emitted was estimated with a Leader 50 luminometer (Gen-Probe Inc., San Diego, CA, USA) and quantified as relative light units (RLUs). Definitive identification was achieved by mycolic acids high-performance liquid chromatography (HPLC) analysis (17). Drug susceptibility pattern was determined in 12B liquid medium (Becton Dickinson Biosciences) by using the radiometric macrodilution method developed for MAC (18).
Six isolates were recovered in a 6-month period. M. celatum was isolated from different specimens: two isolates were from bronchial washing and four from sputum. Acid-fast smears were positive in five of the six specimens. Cultures on Löwenstein-Jensen medium yielded multiple colonies, ranging from 100–150 CFU to a confluent growth. No additional microorganisms were isolated during the admission and follow-up period. Amplification tests performed on all respiratory specimens were repeatedly negative for MTB on smear samples positive and negative for M. celatum. M. celatum strains were isolated on both currently used media, radiometric liquid medium (Bactec 12B) and conventional Löwenstein-Jensen solid medium. We used an extended panel of biochemical and cultural tests for conventional identification. On the basis of these test results, the most likely identification, estimated by a program for the computerized identification of mycobacteria (19), appeared to be M. avium-intracellulare (Table). All strains isolated from this patient produced a weak yellow pigment in the dark, which grew at 45°C and were negative for arylsulfatase activity when tested after 3 days by the method of Kubica and Rigdon (20). The Bactec NAP test (Becton Dickinson Biosciences) did not demonstrate, as expected, any inhibition by para-nitro-α-acetilamino-β-hydroxypropiophenone (NAP). The hybridization test performed with Accuprobe specific for MAC gave negative results. Weak positive or slightly below the cutoff (30,000 RLUs) results were observed with Accuprobe MTB (mean RLU value 51,170; range 28,219–111,365). When selection reagent (unbound probe hydrolysis) incubation time was set at 10 min, M. celatum strains clearly showed negative results (range 5,721–9,574; mean RLU value 7,210). A reference strain (M. tuberculosis ATCC 25177), run in the hybridization assay as a positive control, gave RLUs of 580,321, about 20 times the cut-off value. Finally, the strains were sent to the Mycobacteria Reference Centre in Florence, Italy (Dr. E. Tortoli), and studied by HPLC analysis of mycolic acids. They showed the same profile that allowed all of our strains to be attributed to M. celatum.
MICs (μg/mL) determined for the first isolate were the following: streptomycin, 2.0; isoniazid, 0.5; rifampin, >64.0; ethambutol, 2.5; ciprofloxacin, 1.0; ofloxacin, 1.0; clarithromycin, 1.0; ethionamide, 2.5; and rifabutin, 0.5. Drug susceptibility testing was completed after 6 days.
We searched the English-language literature from 1993 to 2001 for all previously reported cases of pulmonary infections with M. celatum in immunocompetent patients. The search yielded two published reports (12,13). Descriptions of the clinical signs, radiologic findings, and treatment were not always available, however.
Clinical and radiographic features of M. celatum pulmonary infection resemble those of tuberculosis and other nontuberculous mycobacterial infections. Cough and weight loss are the main symptoms, while pulmonary infiltrates and extensive cavitations have been described in all published reports. Patients did not suffer from any underlying diseases when pulmonary infection with M. celatum was diagnosed and had a strongly positive tuberculin skin test, suggesting immunocompetence. Published data and our findings indicate that M. celatum caused pulmonary disease, and not merely colonization, in the affected patients. Indeed, all of these cases met the American Thoracic Society criteria for the diagnosis of nontuberculous mycobacterial disease. The patients displayed radiographic evidence of pulmonary disease that could not be attributed to other causes and that was associated in two of three cases with the repeated isolation of the same mycobacterial strain. By contrast, clinical presentation in immunocompromised patients differs greatly, being characterized by interstitial pulmonary infiltrates or by clinical and micobiologic evidence of disseminated disease (7).
M. celatum strains were isolated on currently used liquid media in all reported cases (radiometric Bactec 12B and MB/BacT [Organon Teknika B.V., Boxtel, the Netherlands]), while samples from one of the published case-patients did not grow on conventional Löwenstein-Jensen solid medium, and growth has not been reported for samples from the second case-patient. Data from an extended panel of biochemical and cultural tests for conventional identification have not been reported; however, the appearance of a scotochromogenic pale yellow pigment with growth at 45°C and a negative 3-day arylsulfatase test are in full agreement with our present and previous findings (7). All strains showed a partial hybridization with Accuprobe MTB, which disappeared after 10 min of incubation with selection reagent. From a practical standpoint, setting the selection time at 10 min is advisable when the Accuprobe identification is performed on a mycobacterial culture grown in a liquid medium. In this situation, the characteristics of mycobacterial colonies cannot be observed and false-positive reactions may lead to misidentification. However, if the Accuprobe assay is carried out on a mycobacterial culture grown on a solid medium, a 5-min selection time is recommended. In this case, the appearance of mycobacterial colonies and their characteristics of growth may help to evaluate Accuprobe results. Thus, a weak positive reaction when testing nontuberculous mycobacteria with the M. tuberculosis complex Accuprobe strongly suggests that the organism is likely to be M. celatum.
In one case (13), a misleading positive reaction occurred when a clinical sample containing M. celatum was tested for MTB by the Amplified M. tuberculosis direct test (AMTD, Gen-Probe, Inc.). The test amplifies 16S rRNA of Mycobacterium species by transcription-mediated amplification (21). The resulting amplicons are then detected by a hybridization protection assay by using a probe that targets the same genomic region as the probe of the Accuprobe kit (22). In our case, when we tested clinical samples by a different amplification system, no false-positive results potentially leading to a delay of appropriate treatment were reported. Patients (including our case-patient) were given different treatment regimens, with three or four antibiotics, mainly ethambutol, rifabutin, clarithromycin, and ciprofloxacin. Clinical improvement, as defined by resolution of symptoms and improved radiographic findings (infiltrates and cavitary lesions), was obtained within 6 months after initiation of therapy in two of three patients. One patient who received antimycobacterial therapy died 10 weeks after admission from complications apparently related to the M. celatum infection (12). Data from necropsy were not reported. Both cured patients showed a marked improvement when clarithromycin was added the chemotherapy regimen; the patient who later died felt better for 3 weeks when his chemotherapy was changed to a new schedule that included clarithromycin. Despite evidence of clinical and radiologic improvement, one patient was still sputum-positive 1 year after the initiation of therapy (13). In-vitro susceptibility data (when available) seemed to correlate with patients’ clinical improvement as the MICs of tested drugs were below the serum concentrations achievable during therapy.
Our findings show that M. celatum is an infrequent cause of potentially treatable pulmonary disease in immunocompetent subjects. Clinical picture and repeated isolation from respiratory specimens enabled us to consider M. celatum strains as clinically relevant in all the patients reported. Treatment with a three- or four-drug combination, including clarithromycin and ethambutol, should result in considerable reduction in the illness associated with this disease. Although at present only 16S rDNA sequencing or mycolic acid HPLC analysis can confirm M. celatum identification, the most practical way to get a preliminary identification relies on a positive DNA hybridization signal for MTB at 5 min but negative hybridization at 10 min with the Accuprobe test. Finally, although no major immunologic disorder could be detected in these patients, the host’s defense failure associated with M. celatum infection suggests a possible “hidden immunodeficiency” rather than a true immunocompetence. Recent data on the immunology of nontuberculous mycobacterial infections (23) seem to support this hypothesis.
Dr. Piersimoni is a clinical microbiology consultant at the Umberto I°–Torrette Hospital in Ancona, Italy. His primary research interests focus on epidemiologic and clinical aspects of mycobacterial infections.
References
- Butler WR, O’Connor SP, Yakrus MA, Smithwick RW, Plikaytis BB, Moss CW, Mycobacterium celatum sp.nov. Int J Syst Bacteriol. 1993;43:539–48. DOIPubMedGoogle Scholar
- Reischl U, Feldmann K, Naumann L, Gaugler BMJ, Ninet B, Hirschel B, 16S rRNA sequence diversity in Mycobacterium celatum strains caused by presence of two different copies of 16S rRNA gene. J Clin Microbiol. 1998;36:1761–4.PubMedGoogle Scholar
- Bull TJ, Shanson DC, Archard LC, Yates MD, Hamid ME, Minnikin DE. A new group (type 3) of Mycobacterium celatum isolated from AIDS patients in the London area. Int J Syst Bacteriol. 1995;45:861–2. DOIPubMedGoogle Scholar
- Butler WR, O’Connor SP, Yakrus MA, Gross WM. Cross-reactivity of genetic probe for detection of Mycobacterium tuberculosis with newly described species Mycobacterium celatum. J Clin Microbiol. 1994;32:536–8.PubMedGoogle Scholar
- Emler S, Ninet B, Rohner P, Auckenthaler R, Jäger D, Hirschel B. Molecular basis for cross-reactivity between a strain of Mycobacterium terrae and DNA probes for Mycobacterium tuberculosis complex. Eur J Clin Microbiol Infect Dis. 1995;14:627–9. DOIPubMedGoogle Scholar
- Tortoli E, Piersimoni C, Bacosi D, Bartoloni A, Betti F, Bono L, Isolation of the newly described species Mycobacterium celatum from AIDS patients. J Clin Microbiol. 1995;33:137–40.PubMedGoogle Scholar
- Piersimoni C, Tortoli E, de Lalla F, Nista D, Donato D, Bornigia S, Isolation of Mycobacterium celatum from patients infected with human immunodeficiency virus. Clin Infect Dis. 1997;24:144–7.PubMedGoogle Scholar
- Garcìa-Garrote F, Ruiz-Serrano MJ, Cosìn J, Alcalà L, Ortega A, Bouza E. Mycobacterium celatum as a cause of disseminated infection in an AIDS patient. Clin Microbiol Infect. 1997;3:583–4. DOIPubMedGoogle Scholar
- Gholizadeh Y, Varnerot A, Maslo C, Salauze B, Badaoui H, Vincent V, Mycobacterium celatum infection in two HIV-infected patients treated prophylactically with rifabutin. Eur J Clin Microbiol Infect Dis. 1998;17:278–81.PubMedGoogle Scholar
- Bonomo RA, Briggs JM, Gross W, Hassan M. Graham RC, Butler R, et al. Mycobacterium celatum infection in a patient with AIDS. Clin Infect Dis. 1998;26:243–5. DOIPubMedGoogle Scholar
- Haase G, Skopnik H, Batge S, Böttger EC. Cervical lymphadenitis caused by Mycobacterium celatum. Lancet. 1994;344:1020–1. DOIPubMedGoogle Scholar
- Bux-Gewehr I, Hagen HP, Rüsch-Gerdes S, Feurle GE. Fatal pulmonary infection with Mycobacterium celatum in an apparently immunocompetent patient. J Clin Microbiol. 1998;36:587–8.PubMedGoogle Scholar
- Tjhie JHT, van Belle AF, Dessens-Kroon M, van Soolingen D. Misidentification and diagnostic delay caused by a false positive amplified Mycobacterium tuberculosis direct test in an immunocompetent patient with a Mycobacterium celatum infection. J Clin Microbiol. 2001;39:2311–2. DOIPubMedGoogle Scholar
- American Thoracic Society. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. Am J Respir Crit Care Med. 1997;156:S1–25.PubMedGoogle Scholar
- Metchock B, Nolte FS, Wallace RJ Jr. Mycobacterium, In Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH, editors. Manual of clinical microbiology, 7th ed. Washington: ASM Press;1999. p.399-437.
- Somoskövi A, Hotaling JE, Fitzgerald M, Jonas V, Stasik D, Parson LM, False-positive results for Mycobacterium celatum with the Accuprobe Mycobacterium tuberculosis complex assay. J Clin Microbiol. 2000;38:2743–5.PubMedGoogle Scholar
- Tortoli E, Bartoloni A, Burrini C, Mantella A, Simonetti MT. Utility of high performance liquid chromatography for identification of mycobacterial species rarely encountered in clinical laboratories. Eur J Clin Microbiol Infect Dis. 1995;14:240–3. DOIPubMedGoogle Scholar
- Siddiqi SH, Heifets LB, Cynamon MH, Hooper NM, Laszlo A, Libonati JP, Rapid broth macrodilution method for determination of MICs for Mycobacterium avium isolates. J Clin Microbiol. 1993;31:2332–8.PubMedGoogle Scholar
- Tortoli E, Boddi V, Penati V. Development and evaluation of a program and probability matrix for the computer-aided identification of non-tuberculous mycobacteria. Binary Computed Microbiology. 1992;4:200–4.
- Kubica GP, Rigdon AL. The arylsulfatase activity of acid-fast bacilli. III. Prelimina investigation of rapidly growing acid-fast bacilli. Am Rev Respir Dis. 1961;83:737–40.PubMedGoogle Scholar
- Boddinghaus B, Rogall T, Flohr H, Blocker H, Böttger EC. Detection and identification of mycobacteria by amplification of rRNA. J Clin Microbiol. 1990;28:1751–9.PubMedGoogle Scholar
- Bodmer T, Gurtner A, Schopfer K, Matter L. Screening of respiratory tract specimens for the presence of Mycobacterium tuberculosis by using the Gen-Probe amplified Mycobacterium tuberculosis direct test. J Clin Microbiol. 1994;32:1483–7.PubMedGoogle Scholar
- Casanova J-L, Abel L. Genetic dissection of immunity to mycobacteria: the human model. Annu Rev Immunol. 2002;20:581–620. DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleTable of Contents – Volume 9, Number 3—March 2003
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Claudio Piersimoni, Department of Clinical Microbiology, Umberto I°–Torrette Hospital, via Conca, I-60020 Ancona, Italy; fax: +39 071 596.4184
Top