Volume 16, Number 5—May 2010
Research
Effects of Pneumococcal Conjugate Vaccine 2 Years after Its Introduction, the Netherlands
Figure 5
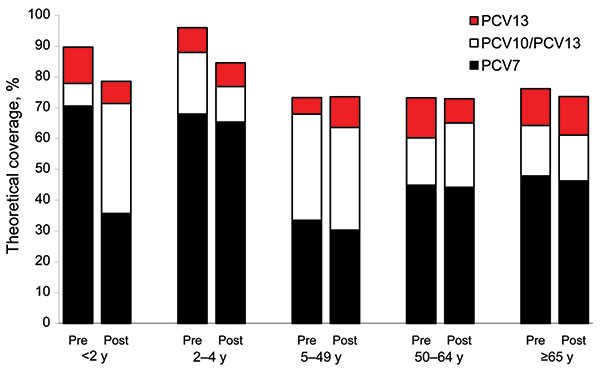
Figure 5. Age group–specific theoretical coverage of pneumococcal conjugate vaccines during the preimplementation and postimplementation periods of 7-valent pneumococcal conjugate vaccine (PCV-7), the Netherlands. IPD, invasive pneumococcal disease; PCV-10/PCV13, additional coverage by PCV-10 and PCV-13; PCV-13, additional coverage by PCV-13 alone; pre, preimplementation period (June 2004–June 2006); post, postimplementation period (June 2006–June 2008).
Page created: December 23, 2010
Page updated: December 23, 2010
Page reviewed: December 23, 2010
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.