Volume 8, Number 3—March 2002
Dispatch
Rickettsia felis in Ctenocephalides spp. Fleas, Brazil
Figure 2
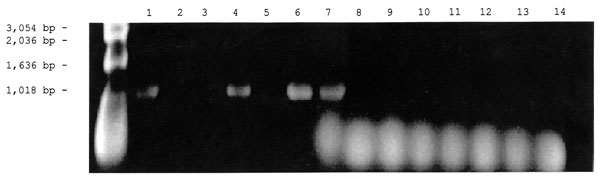
Figure 2. Detection of the Rickettsia specific 17-kDa gene by polymerase chain reaction amplification in DNA extracted from ticks and fleas. The vectors were first placed in 1.5-mL microcentrifuge tubes containing 200 µL of 10 mM phosphate-buffered saline, pH 7.4, and were crushed with a micropestle. The suspensions were lysed in 0.5% sodium dodecyl sulfate and incubated with 100 µg/mL proteinase K at 37°C for 1 hour in the case of fleas or overnight in the case of ticks. The lysed suspensions were extracted twice with an equal volume of phenolchloroform, followed by a single chloroform extraction. The extracted DNA was amplified with primer 1 (5′-GCTCTTGCAACTTCTATGTT-3′) and primer 2 (5′-CATTGTTCGTCAGGTTGGCA-3′) as described by Webb et al. (10) for amplification of a 434-bp fragment from the rickettsial 17-kDa protein gene. PCR was performed at 30 cycles for 1 minute at 94°C, 5 minutes at 48°C, and 2 minutes at 72°C. The PCR products were then separated by electrophoresis in 1% agarose gel and stained with ethidium bromide. Lanes 1-3: DNA from cat fleas, Lanes 4-6: DNA from dog fleas, Lane 7: 17- kDa gene Rickettsia felis DNA (Positive Control), Lanes 8-14: DNA from ticks.
References
- Piza JT. Considerações epidemiológicas e clínicas sobre o Tifo Exantemático de São Paulo. São Paulo: Sociedade Impressora Paulista; 1932. p. 11-119.
- Monteiro JL, Fonseca F. Typho endêmico de S. Paulo. Novas experiências sobre a transmissão experimental por carrapatos (Boophilus microplus e Amblyomma cajennense). Mem Inst Butantan (São Paulo). 1932;10:33–50.
- Travassos J, Rodrigues PM, Carrijo LN. Tifo murino em S. Paulo. Identificação da Rickettsia mooseri isolada de um caso humano. São Paulo: Mem Inst Butantan; 1949.
- Azad AF, Sacci JB Jr, Nelson WM, Dasch GA, Schmidtmann ET, Carl M. Genetic characterization and transovarial transmission of a typhus-like rickettsia found in cat fleas. Proc Natl Acad Sci U S A. 1992;89:43–6. DOIPubMedGoogle Scholar
- Schriefer ME, Sacci JB Jr, Taylor JP, Higgins JA, Azad AF. Murine typhus: updated roles of multiple urban components and a second typhus-like rickettsia. J Med Entomol. 1994;31:681–5.PubMedGoogle Scholar
- Higgins JA, Sacci JB, Schriefer ME, Endris RG, Azad AF. Molecular identification of rickettsia-like microorganisms associated with colonized cat fleas (Ctenocephalides felis). Insect Mol Biol. 1994;3:27–33.PubMedGoogle Scholar
- Zavala-Velazquez JE, Ruiz-Sosa JA, Sanchez-Elias RA, Becerra-Carmona G, Walker DH. Rickettsia felis rickettsiosis in Yucatan. Lancet. 2000;356:1079–80. DOIPubMedGoogle Scholar
- Raoult D, La Scola B, Enea M, Fournier P-E, Roux V, Fenollar F, A flea-associated rickettsia pathogenic for humans. Emerg Infect Dis. 2001;7:73–81.PubMedGoogle Scholar
- Bouyer DH, Stenos J, Crocquet-Valdes P, Moron CG, Popov VL, Zavala-Velazquez JE, Rickettsia felis: molecular characterization of a new member of the spotted fever group. Int J Syst Evol Microbiol. 2001;51:339–47.PubMedGoogle Scholar
- Webb L, Carl M, Malloy DC, Dasch GA, Azad AF. Detection of murine typhus infection in fleas by using the polymerase chain reaction. J Clin Microbiol. 1990;28:530–4.PubMedGoogle Scholar
- Williams SG, Sacci JB Jr, Schriefer ME, Andersen EM, Fujioka KK, Sorvillo FJ, Typhus and typhus like rickettsiae associated with opossums and their fleas in Los Angeles County, California. J Clin Microbiol. 1992;30:1758–62.PubMedGoogle Scholar
- Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988;16:10881–90. DOIPubMedGoogle Scholar
- Galvão MAM. Febre maculosa em Minas Gerais: um estudo sobre a distribuição da doença no Estado e seu comportamento em área de foco peri-urbano. Belo Horizonte: Faculdade de Medicina da UFMG; 1996. p. 114 (PhD Thesis, Tropical Medicine).
- Galvão MAM, Chamone CB, Calic SB, Machado MC, Otoni MEA, Dietze R, Serologic evidence of spotted fever group Rickettsia in Novo Cruzeiro Municipality-Minas Gerais State, Brazil. In: Raoult D, Brouqui P, editors. Rickettsial diseases at the turn of the third millennium. Marseille: Elsevier; 1999. p. 240-3.
- Galvão MAM, Mafra CL, Oliveira R, Chamone CB, Calic SB, Walker DH. Clinical and laboratorial evidence of Rickettsia felis in Latin America. Proceedings of the American Society for Rickettsiology—Bartonella as an Emerging Pathogen Group 2001 Joint Conference, Big Sky [URL: www.cas.umt.edu/rickettsiology/]. Montana. 2001; (
Aug ):17–22.