Volume 10, Number 4—April 2004
Dispatch
Anaplasma phagocytophilum, Babesia microti, and Borrelia burgdorferi in Ixodes scapularis, Southern Coastal Maine
Cite This Article
Citation for Media
Abstract
Ixodes scapularis (deer ticks) from Maine were tested for multiple infections by polymerase chain reaction and immunofluorescence. In 1995, 29.5%, 9.5%, and 1.9% of deer ticks were infected with Borrelia burgdorferi, Anaplasma phagocytophilum, and Babesia microti, respectively. In 1996 and 1997, the number of A. phagocytophilum-infected ticks markedly declined. In 1995 through 1996, 4 (1.3%) of 301 were co-infected.
Throughout its range in the eastern and upper midwestern United States, Ixodes scapularis (Ixodes dammini) (deer tick) is the vector of Borrelia burgdorferi, the causative agent of Lyme disease. In recent decades, it has been associated with several other pathogens, including bacteria, viruses, and protozoa, a guild of pathogens similar to that seen in the related tick Ixodes ricinus in Europe (1).
I. scapularis was determined to be the vector of the intraerythrocytic protozoan Babesia microti on Nantucket Island, Massachusetts in 1979 (2). Human granulocytic ehrlichiosis (HGE) was first described in 1994 in patients from Wisconsin and Minnesota (3). I. scapularis was determined to be a competent vector of the obligate intracellular bacteria that cause HGE, and field-derived ticks from Massachusetts were found to be co-infected with the HGE agent and B. burgdorferi (4). The agent of HGE, previously referred to as Ehrlichia phagocytophila, has recently been reclassified as Anaplasma phagocytophilum (5).
Rodents and birds have been demonstrated to be reservoirs of the Lyme disease spirochete in areas of Maine where the tick is established (6). This study sought to determine if I. scapularis at the northern edge of its range was infected with A. phagocytophilum and Ba. microti, in addition to B. burgdorferi.
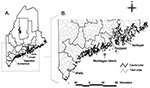
I. scapularis nymphs and adult females that had partially fed on a variety of hosts were collected in 1995 through 1997 from coastal areas in Maine, from York to Hancock counties, where the tick is established and Lyme disease is endemic (Figure A). Ticks removed from pets and humans were submitted to our laboratory for species confirmation. Nymphs were also removed from white-footed mice and eastern chipmunks live-trapped on established research grids in the town of Wells and from Norway rats trapped on an offshore island. Mammal trapping procedures were approved by the Maine Medical Center Institutional Animal Care and Use Committee. One I. scapularis female was removed from a nontranquilized, live, white-tailed deer that had become accustomed to humans on Monhegan Island. All ticks were transported alive to the laboratory.
Ticks were dissected on sterile glass slides in a drop of 10 mmol Tris-HCl, 1 mmol EDTA pH 8 (TE). Salivary glands were isolated, and one gland from each tick was stained by the Feulgen reaction for microscopic examination for inclusions (7); the other gland was prepared for DNA extraction. A smear of tick midgut was prepared for fluorescent microscopic examination for spirochetes as described previously (6).
All polymerase chain reaction (PCR) tests were performed on salivary glands from individual ticks except for 14 instances in 1995 when salivary glands from several ticks collected from an individual host were pooled for PCR analysis. For statistical purposes, when a PCR product was obtained from a pool of salivary glands from multiple ticks, only one tick in the pool was assumed to be infected.
Salivary glands were stored at –20°C in 50 μL of TE buffer until DNA extraction. DNA was isolated using a standard phenol/chloroform extraction procedure (8) or by using the IsoQuick kit (ORCA Research, Bothell, WA) according to the manufacturer’s protocol and placed in 20 μL of TE buffer. Sterile aerosol-barrier tips were used during all procedures. DNA isolation and PCR reactions were performed in separate laboratories. Positive and negative controls were included in each PCR reaction.
Babesia was detected by amplifying a 437-bp portion of the eukaryotic 18S rRNA gene by PCR using primer pair PiroA/PiroB (9). Components were denatured at 94°C for 45 sec, annealed at 60°C for 45 sec, and extended at 72°C for 2 min, for a total of 40 cycles. Samples were separated by electrophoresis on a 1% Sea Plaque agarose gel containing ethidium bromide and 40 mmol Tris-acetate 1 mmol EDTA pH 8.3 buffer.
Anaplasma was identified by the amplification of 16S rDNA by PCR. The primer pair consisting of GE9 (3) and Ehr747 (10) was used to generate an 849-bp fragment. The thermal cycling profile used was the same as for Babesia.
Amplified products were excised from the gels, treated with Beta-agarase (Sigma, St. Louis, MO), cycle-sequenced using dye-labeled dideoxy terminators (Applied Biosystems Big Dye Reaction Kit, Foster City, CA) and purified by using Centri-Sep columns (Princeton Separations, Adelphia, NJ). Samples were electrophoresed on a 6% polyacrylamide stretch gel using an ABI 373A DNA sequencer. DNA sequences were compared with previously published sequences for species identification, using the Sequence Navigator program by Applied Biosystems.
From 1995 to 1997, PCR was performed on salivary glands from 223 I. scapularis nymphs and 171 females. Nymphs made up 44% of ticks tested the first year of the study and 61% in both of the later years. Table presents the prevalence of infection with A. phagocytophilum, Ba. microti, and B. burgdorferi in I. scapularis studied each year.
Four of the positive PCR results were obtained from pooled glands. Assuming only one gland in each pool was positive, a total of six nymphs (possible range 6–12) and five female I. scapularis (possible range 5–7) were infected with A. phagocytophilum. Ba. microti was found in two nymphs and one female tick. Nine of the infected ticks were collected in the town of Wells in York County, three were from Monhegan Island in Lincoln County, and one each was from the towns of Rockport in Knox County and Northport in Waldo County (Figure B). Four nymphs were infected with two organisms (Table). All of the co-infected ticks were from the town of Wells in York County.
Babesia spp. piroplasms were microscopically visualized by the Feulgen reaction in salivary acini from 21 ticks. Two glands positive for Babesia spp. by visual inspection had PCR product that matched sequences for Ba. microti; the remaining 19 (90.5%) of 21 samples matched sequences for Ba. odocoilei, a parasite of deer not known to cause human illness (9). Two (18%) of 11 feulgen-stained glands from ticks determined to be positive for A. phagocytophilum by PCR were considered positive by visual inspection of the other gland. All amplification product from the A. phagocytophilum–positive ticks had 99% homology (848/849 bp) with sequences of E. phagocytophila-human agent of Chen et al. (GenBank accession no. U02521) (3).
Multiple studies conducted in hyperendemic areas of Lyme disease have reported A. phagocytophilum and Ba. microti in field-collected I. scapularis (4,7,10–13). Schwartz et al. reported an increase in the percent of adult deer ticks infected with the agent of HGE in Westchester County, New York from 32% of ticks collected in1984 and tested retrospectively, to 53% in 1995 (11). In a 2-year study in Connecticut, 12.5% of adult ticks in 1996 and 19% in 1997 were infected with A. phagocytophilum (12). The current study showed a decrease in the percent of infected ticks collected from the same geographic areas for a 3-year period. A. phagocytophilum infection rates declined from 9.5% in 1995 to 0.5% and 0% in subsequent years. The percent of ticks infected with B. burgdorferi remained relatively constant for the 3-year period (Table).
Ba. microti infection rates based on DNA sequences of the organism have been reported from 5% of adult ticks tested in New Jersey (13) to 9% of adult ticks on Nantucket Island in Massachusetts (4). In 1995, 1.9% of ticks tested in this study were positive for Ba. microti; the percent infected dropped in subsequent years to 0.5% and 0%. This low prevalence of Ba. microti infection in Maine ticks is not unexpected. Mather et al. reported that Ba. microti was found only in areas of Rhode Island where tick abundance reached >20 nymphs per hour of flagging (14). In our study, the three ticks infected with Ba. microti were collected in the town of Wells in coastal York County where tick density is the highest in the state (unpub. data). Although enzootic Ba. microti maintained by Ixodes angustus or other nidicolous ticks may be widespread in Maine, I. scapularis density high enough to support zoonotic transmission of Ba. microti may only occur in a few foci (15).
That the prevalence of infection of ticks with B. burgdorferi during this 3-year study remained fairly constant while that of A. phagocytophilum showed greater variation is of interest. Other researchers have shown that white-footed mice remain reservoir competent for A. phagocytophilum for short periods of time (16) and that transmission of multiple organisms may have a different dynamic than that of single pathogens (17). Few studies have followed the natural infection of tick hosts with multiple organisms over time. This study indicates that the prevalence of these emerging pathogens may not be as stable from year to year as is the rodent-I. scapularis-B. burgdorferi cycle.
This study provides evidence of the potential for human exposure to multiple tick-borne pathogens in southern coastal Maine and that the risk for exposure to A. phagocytophilum may vary considerably from year to year.
Ms. Holman is a research associate in the Vector-borne Disease Laboratory of the Maine Medical Center Research Institute. She has spent the last 9 years investigating tick-borne disease in Maine.
Acknowledgments
We gratefully acknowledge Sam R. Telford III for his helpful advice and for a continuing supply of fluorescent antibody, the management of the Wells National Estuarine Research Reserve for permission to conduct research studies at the Reserve, and the residents of Monhegan Island and the Monhegan Associates for their continuing assistance and cooperation.
This study was supported by Contract Number 200-91-0915 and Cooperative Agreement Number U50/CCU114672 both from the Centers for Disease Control and Prevention and by Contract Number 013-10A-2504-03 from the Bureau of Health of the Maine Department of Human Services.
References
- Telford SR Iii, Dawson JE, Halupka KC. Emergence of tickborne diseases. Sci Med (Phila). 1997;4:24–33.
- Spielman A, Clifford CM, Piesman J, Corwin MD. Human babesiosis on Nantucket Island, USA: Description of the vector, Ixodes (Ixodes) dammini, n. sp. J Med Entomol. 1979;15:218–34.PubMedGoogle Scholar
- Chen SM, Dumler JS, Bakken JS, Walker DH. Identification of a granulocytic Ehrlichia species as the etiologic agent of human disease. J Clin Microbiol. 1994;32:589–95.PubMedGoogle Scholar
- Telford SR III, Dawson JE, Katavolos P, Warner CK, Kolbert CP, Persing DH. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc Natl Acad Sci U S A. 1996;93:6209–14. DOIPubMedGoogle Scholar
- Dumler JS, Barbet AF, Bekker CP, Dasch GA, Palmer GH, Ray SC, Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int J Syst Evol Microbiol. 2001;51:2145–65.PubMedGoogle Scholar
- Rand PW, Lacombe EH, Smith RP Jr, Ficker J. Participation of birds (Aves) in the emergence of Lyme disease in southern Maine. J Med Entomol. 1998;35:270–6.PubMedGoogle Scholar
- Piesman J, Mather TN, Donahue JG, Levine J, Campbell JD, Karakashian SJ, Comparative prevalence of Babesia microti and Borrelia burgdorferi in four populations of Ixodes dammini in eastern Massachusetts. Acta Trop. 1986;43:263–70.PubMedGoogle Scholar
- Caporale DA, Kocher TD. Sequence variation in the outer-surface protein genes of Borrelia burgdorferi. Mol Biol Evol. 1994;11:51–64.PubMedGoogle Scholar
- Armstrong PM, Katavolos P, Caporale DA, Smith RP, Spielman A, Telford SR III. Diversity of Babesia infecting deer ticks (Ixodes dammini). Am J Trop Med Hyg. 1998;58:739–42.PubMedGoogle Scholar
- Pancholi P, Kolbert CP, Mitchell PD, Reed KD, Dumler JS, Bakken JS, Ixodes dammini as a potential vector of human granulocytic ehrlichiosis. J Infect Dis. 1995;172:1007–12.PubMedGoogle Scholar
- Schwartz I, Fish D, Daniels TJ. Prevalence of the rickettsial agent of human granulocytic ehrlichiosis in ticks from a hyperendemic focus of Lyme disease. N Engl J Med. 1997;337:49–50. DOIPubMedGoogle Scholar
- Levin ML, des Vignes F, Fish D. Disparity in the natural cycles of Borrelia burgdorferi and the agent of human granulocytic ehrlichiosis. Emerg Infect Dis. 1999;5:204–8. DOIPubMedGoogle Scholar
- Varde S, Beckley J, Schwartz I. Prevalence of tick-borne pathogens in Ixodes scapularis in a rural New Jersey County. Emerg Infect Dis. 1998;4:97–9. DOIPubMedGoogle Scholar
- Mather TN, Nicholson MC, Hu R, Miller NJ. Entomological correlates of Babesia microti prevalence in an area where Ixodes scapularis (Acari: Ixodidae) is endemic. J Med Entomol. 1996;33:866–70.PubMedGoogle Scholar
- Goethert HK, Lubelczyk C, Lacombe E, Holman M, Rand P, Smith RP, Enzootic Babesia microti in Maine. J Parasitol. 2003;89:1069–71. DOIPubMedGoogle Scholar
- Stafford KC, Massung RF, Magnarelli LA, Ijdo JW, Anderson JF. Infection with agents of human granulocytic ehrlichiosis, Lyme disease, and babesiosis in wild white-footed mice (Peromyscus leucopus) in Connecticut. J Clin Microbiol. 1999;37:2887–92.PubMedGoogle Scholar
- Levin ML, Fish D. Interference between the agents of Lyme disease and human granulocytic ehrlichiosis in a natural reservoir host. Vector Borne Zoonotic Dis. 2001;1:139–48. DOIPubMedGoogle Scholar
Figure
Table
Cite This Article1Dr. Caporale was working at the University of Maine at Orono at the time of the study. She is currently at the Department of Biology, University of Wisconsin–Stevens Point, Stevens Point, WI.
Table of Contents – Volume 10, Number 4—April 2004
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Mary S. Holman; Vector-borne Disease Laboratory, Maine Medical Center Research Institute, 13 Charles Street, 3rd Floor, Portland, ME 04102, USA; fax 207-842-7147
Top