Volume 10, Number 9—September 2004
Research
Methicillin-resistant Staphylococcus aureus in Europe, 1999–2002
Cite This Article
Citation for Media
Abstract
We explored the variation in proportions of methicillin-resistant Staphylococcus aureus (MRSA) between and within countries participating in the European Antimicrobial Resistance Surveillance System and temporal trends in its occurrence. This system collects routine antimicrobial susceptibility tests for S. aureus. We examined data collected from January 1999 through December 2002 (50,759 isolates from 495 hospitals in 26 countries). MRSA prevalence varied almost 100-fold, from <1% in northern Europe to >40% in southern and western Europe. MRSA proportions significantly increased in Belgium, Germany, Ireland, the Netherlands, and the United Kingdom, and decreased in Slovenia. Within countries, MRSA proportions varied between hospitals with highest variance in countries with a prevalence of 5% to 20%. The observed trends should stimulate initiatives to control MRSA at national, regional, and hospital levels. The large differences between hospitals indicate that efforts may be most effective at regional and hospital levels.
Staphylococcus aureus is an important cause of community- and hospital-acquired infections. Infections caused by methicillin- or oxacillin-resistant S. aureus (MRSA) are mainly nosocomial and are increasingly reported from many countries worldwide (1). As MRSA strains are frequently resistant to many different classes of antimicrobial drugs, second- and third-line antimicrobial resistance is a growing concern (2). Surveillance of MRSA provides relevant information on the extent of the MRSA epidemic, identifies priorities for infection control and the need for adjustments in antimicrobial drug policy, and guides intervention programs (3).
In Europe, several surveillance systems collect data on MRSA (4,5). Most collect data from specific types of hospitals, for certain periods, or information related to specific antimicrobial susceptibility patterns. The only ongoing initiative that continuously monitors antimicrobial resistance in most European countries is the European Antimicrobial Surveillance System (EARSS), funded by Directorate General for Health and Consumer Protection of the European Commission. This network connects national surveillance systems and provides comparable and validated results of routine antimicrobial susceptibility tests (AST) following standardized protocols from a representative set of laboratories per country (6). Timely and detailed feedback is given through a freely accessible and interactive Web site (http:\\www.earss.rivm.nl). EARSS was established in 1998 and currently connects >600 laboratories in 28 countries, which serve >100 million people. Preliminary EARSS results showed considerable differences in the proportions of MRSA across Europe (7,8).
We report results of antimicrobial susceptibility testing of S. aureus blood isolates from 1999 to 2002 in Europe; these results show variation in the prevalence of MRSA, including variation in its proportions at the hospital level. To assess recent changes in the epidemiology of MRSA within countries, we also present country-specific temporal trends in the occurrence of MRSA.
Data Collection
Data (identification number of isolate, EARSS laboratory code, date and type of specimen, sex and age of patient, EARSS hospital code, hospital ward to which patient is admitted, result of mecA gene polymerase chain reaction [PCR], and susceptibility to several antimicrobial drugs, including oxacillin and vancomycin) are collected through national surveillance systems. AST results of every first S. aureus blood isolate per patient per quarter are submitted to the EARSS database by national data managers. After authorization by the national representatives by using standard feedback reports, national data are included in the EARSS database and become available on the Web site.
Susceptibility Testing
Antimicrobial susceptibility is tested according to a standardized protocol (5). Briefly, laboratories report oxacillin susceptibility, preferably determined by an oxacillin-screening plate or an oxacillin disk-diffusion test. To confirm methicillin resistance, the minimum inhibitory concentration (MIC) for oxacillin or the presence of mecA gene by PCR is determined. Reporting vancomycin MIC is recommended for MRSA isolates.
Interpretative AST results (i.e., sensitive [S], intermediate [I], and resistant [R], in accordance with defined guidelines) are accepted. Most (71%) of the laboratories have adopted the guidelines of the National Committee for Clinical Laboratory Standards (NCCLS; www.nccls.org). Most guidelines agree that S. aureus isolates should be considered nonsusceptible (R) to oxacillin if the MIC is ≥4 mg/L. Lower MIC breakpoints (R if MIC >2 mg/L) are only suggested by the Deutsche Industrie-Norm (DIN) (www.din.de) and guidelines of the Swedish Reference Group for Antibiotics (SRGA) (www.srga.org).
Data Analysis
We rejected observations lacking mandatory information (i.e., laboratory code, date of specimen, either patient identification number or month and year of birth, pathogen code, antibiotic code, or test result [S or R]); duplicate records and repeat isolates from the same patient were also rejected. Isolates with an interpretative AST result of “R” (resistant) to oxacillin or one of its equivalents (cloxacillin, dicloxacillin, and flucloxacillin) were defined as MRSA. Isolates with intermediate susceptibility were not counted as MRSA and were excluded from the analyses. MRSA proportions were calculated as the number of MRSA isolates divided by the total number of S. aureus isolates obtained from blood cultures.
For the current analysis, data collected from January 1999 through December 2002 were used. We included only information from hospitals with data for at least 20 isolates from countries reporting >100 isolates. To calculate time trends for analyses of variation between hospitals, we included only those hospitals that had participated in at least 3 consecutive years.
Univariate analyses were performed by using chi-square or t tests if appropriate. Country-specific trends in the occurrence of MRSA over time were analyzed by using a multivariate Poisson regression model adjusting for autocorrelation in hospitals (e.g., attributable to possible similarity in blood culturing and AST practice). We also compared countries with respect to variation between hospitals, expressed as the variance in hospital-specific MRSA proportions. To eliminate the natural dependency between variance and mean, the MRSA ratio was first transformed by power (Box-Cox) transformation according to the following formula: T(k/n) = (k/n)λ, where T is the transformed MRSA ratio, k/n is the resistance rate (i.e., the number of resistant isolates divided by the total number of isolates), and λ was chosen in such a way that variance was independent of the mean, i.e., λ = 0.397. The variance was further adjusted by size (in terms of number of isolates reported) of individual hospitals. Country-specific variances were then graphically displayed and compared.
From January 1999 through December 2002, EARSS received AST results of 53,264 S. aureus blood isolates from 27 countries (Norway does not report S. aureus data), including 628 laboratories serving 896 hospitals. Twenty-six countries reported AST results of >100 isolates. The current study included 50,759 isolates from 428 laboratories serving approximately 500 hospitals. Overall, 20% of these isolates were reported as methicillin resistant. A total of 295 hospitals (35,921 isolates, 19 countries) provided data for at least 3 consecutive years and were included in the time trend analyses. Table 1 describes the main characteristics of the data and the proportion of MRSA by country.
MRSA was more frequently isolated from men (21%) than from women (18%, p < 0.001). Patients with a blood culture positive for MRSA were older than patients with methicillin-susceptible S. aureus (MSSA) (mean age, 65.3 [SD 18.7] versus 58.6 [23.4], p < 0.001). The proportion of MRSA was highest among patients admitted to intensive care units (35%).
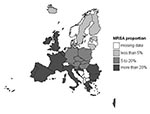
Geographic variation is displayed in Figure 1, which shows a north-south gradient, with the lowest MRSA prevalence in northern Europe and highest prevalence in southern Europe, Israel, the United Kingdom, and Ireland. MRSA proportions varied almost 100-fold, with the lowest proportion in Iceland (0.5%) and the highest proportion in Greece (44%, Table 1).
Statistical analyses of country-specific time trends by Poisson regression (Table 2) showed that increases in MRSA proportions were significant in Belgium (from 22% in 1999 to 27% in 2002), Ireland (39%–45%), Germany (9%–19%), the Netherlands (0.4%–1%) and the United Kingdom (31%–45%). The proportion of MRSA decreased significantly in Slovenia only, from 22% in 2000 to 15% in 2002. The model had difficulties in estimating changes in MRSA proportion in countries with low counts of MRSA isolates, which is reflected in the very wide confidence intervals for Iceland and Bulgaria (Table 2). Relatively large year-to-year fluctuations occurred in some countries (Bulgaria, Greece, Luxembourg, Malta, and Portugal); some of these countries (Bulgaria, Luxembourg, and Malta) had low isolate counts (Table 1). Figure 2 presents significant time trends by showing MRSA proportions per country per year for 1999 through 2002.
Figure 3A shows regional variation in MRSA proportions within countries. Particularly high variation was identified among hospitals in Belgium, the Czech Republic, Spain, Greece, Italy, Portugal, and the United Kingdom. After applying the power transformation, the remaining variation was highest in Germany (Figure 3B), with a variance after transform of 17%. Other countries with relatively high variation in MRSA proportions (variance after transform >15%) between hospitals were Poland, the Czech Republic, and Slovakia. The highest relative variation was found in countries with MRSA proportions from 5% to 20%, with the exception of Hungary and Slovenia. A relatively high variation between hospitals was also found in countries with MRSA proportions >25%. The lowest variation between hospitals was observed for Slovenia (variance after transform, 3%), and variation was also low in France (variance after transform, 5%).
Vancomycin resistance did not occur. Intermediate susceptibility of S. aureus (VISA) was only reported for five isolates from France in 2001.
This is the first EARSS report on the prevalence of MRSA among blood isolates in 27 countries in the European region. We found that proportions of MRSA vary largely across Europe, with the highest proportions in southern and parts of western Europe and lowest proportions in northern Europe. MRSA proportions seem to be increasing in many countries. Significant increases were found for Belgium, Germany, the Netherlands, Ireland, and the United Kingdom, whereas the proportion of MRSA decreased in Slovenia. In all countries, variation between hospitals was observed. The variation between hospitals was highest in Germany and in most other countries with an MRSA prevalence of 5% to 20%. The lowest variation between hospitals was found in Slovenia.
Our results show the European situation with respect to the occurrence of MRSA in blood isolates and confirm other observations (9–11) on invasive isolates; they are also in accordance with findings of other studies with respect to demographic variables, such as sex, age, and patient ward (9,12). Although blood isolates represent the minority of clinically relevant samples, they are indicative of infection. Studies that report MRSA proportions from all sources usually include screening samples that are subject to bias because of differential screening practices. Considering hospital-acquired MRSA only seems to provide insight into the European MRSA epidemic, as the prevalence of community-acquired MRSA in Europe remains very low (0.03%–1.5%), even in countries with a high MRSA prevalence in hospitals (13–17). EARSS provides comparable data, annually validated through external quality assurance exercises, which have repeatedly confirmed a good-to-excellent concordance for identifying MRSA (18).
EARSS accepts susceptibility data according to clinical breakpoints (S, I, R) in agreement with international guidelines. Methicillin resistance is usually defined as having an MIC of >4 mg/L. Because of lower breakpoints (MIC >2 mg/L) defined by SRGA and DIN, this definition may have caused partial overestimation of MRSA proportions reported from Sweden (where SRGA is used in 100% of laboratories), and from Germany (where DIN is used in 59% of the participating laboratories) in comparison to other countries (19). However, most MRSA strains show high-level resistance to oxacillin, although low-level resistant strains are emerging (20). Moreover, such misclassification is unlikely to bias the country-specific temporal trends reported here. In all other countries, all laboratories agree on a single breakpoint (>4 mg/L).
We used Poisson regression modeling adjusting for autocorrelation within hospitals to test for possible time trends in MRSA proportions. This model assumes that the epidemic runs according to an S-curve (21). The results of this analysis need to be interpreted with caution, as confidence intervals are wide, especially for countries with a low number of isolates. Year-to-year fluctuations found for some countries were probably not due to changes in the case-mix, as analyses were performed on data from a constant set of hospitals in each country, but were possibly caused by random variation of low numbers of isolates (Bulgaria, Luxembourg, Malta). Since the model estimates time trends over the 4-year observation period, it did not account for such fluctuations, which should be possible by autoregressive moving average (ARIMA) modeling (22). However, ARIMA modeling requires at least 60 data points, which cannot be provided at this stage.
The temporal increase we found for Germany is supported by a national surveillance study carried out at regular intervals, which reported an increase of MRSA from 2% in 1992 to 21% in 2001 (23,24). Our results for the United Kingdom show that the increase in MRSA proportions, reported from 1992 through 1998 (25,26), continued until 2001, and now appears to have leveled off. This development in the MRSA epidemic reflects the curve of the number of hospitals affected by MRSA outbreaks over time, as predicted by Austin and Anderson (21). The same epidemic curve might apply to Ireland, although the stabilizing MRSA prevalence may also be the result of a nationwide infection control campaign (27). This Strategy for the Control of Antimicrobial Resistance in Ireland (SARI) follows a multidisciplinary approach, focusing on surveillance of antimicrobial resistance and use as well as infection control and stewardship of antibiotic use in the community and in hospitals. National MRSA guidelines are being updated, and the deficit in hospital staffing (laboratory surveillance scientists, infection control nurses, clinical microbiologists, and clinical pharmacists) is currently being addressed (28). In England, several recent initiatives have the goals of increasing awareness and encouraging efforts to control MRSA by individual hospitals. First, a mandatory surveillance program for MRSA bacteremia was launched, which included publication of MRSA diagnoses by named national health trust (29,30). Second, a strategy was published to reduce healthcare-associated infection in England (31), which included guidelines for good hospital practice. The rise in MRSA prevalence in the Netherlands might be the result of the increase in heterogeneously resistant clones with low MICs for oxacillin (4–24 mg/L) (32). The effects of national infection control campaigns launched in Slovenia (J. Kolman, pers. comm.) may have had an impact. With the continuation of EARSS, we will be able to monitor any effect of such campaigns.
Variations in MRSA proportions between hospitals within the same country have been reported (9,33–35), but to our knowledge, this is the first attempt to quantify variation between hospitals at the national level in a European study. We showed that considerable variation in MRSA proportions exists not only between countries but also between hospitals within a country. Regional variation might be explained by different phenomena. The emergence of MRSA is largely due to dissemination of clonal strains, and temporary hospital outbreaks are typically due to clonal expansion (36). If stringent control measures are taken to prevent further MRSA transmission, MRSA prevalence might subsequently be reduced to sporadic levels (12). However, the effectiveness of MRSA control depends on several factors, such as the existence and correct application of hygiene protocols to prevent transmission (hand hygiene, isolation practices, cohorting), level of care needed by patients (indicating host susceptibility), and antimicrobial drug prescription policies (which would influence selective pressure), which might differ between hospitals in a country (37). As Kotilainen and colleagues showed, quick and adequate measures at the hospital level, as well as at the regional level, may be successful in containing the MRSA epidemic (38). Regional variation may also be explained by differences in diagnostic practice and culturing activity and random errors, which may artificially increase variation (39). Also, a differential case-mix attributable to differences in the level of care provided per hospital and differential referral practice may confound our estimates (9,35,40). However, unusually high variation in MRSA proportions between hospitals seems to occur most often in countries experiencing a current surge of MRSA. In support of this hypothesis, in general, MRSA proportions varied most in countries with increasing and intermediate (5%–20%) MRSA prevalence. These countries might have changed from equilibrium with adequate control and elimination of sporadic MRSA and might be on the verge of becoming disease-endemic. This stage may be characterized by abandoning strict search-and-control strategies and adopting more flexible approaches, as happened in England when MRSA prevalence was increasing in the 1990s (41,42). However, MRSA proportions were not increasing in all these countries, and variation in prevalence between hospitals was also high in countries with a high overall MRSA prevalence (>25%) (37). In contrast, in Slovenia, where MRSA proportions have decreased recently, variation between hospitals was low. Thus, the national campaign on infection control might have decreased not only MRSA prevalence but also the variation in MRSA proportions between hospitals.
Our database did not show vancomycin resistance; a few VISA isolates were reported from France only. This finding might be explained by the fact that EARSS collects routine data, whereas VISA will only be detected in specialized laboratories. Moreover, the clinical and epidemiologic importance of (heterogeneous) VISA remains to be clarified.
EARSS results show that MRSA proportions increased in several countries. Variation in MRSA proportions exists at international and at national levels, and regional variation seems to be highest in countries with intermediate MRSA proportions (5%–20%). Although the reasons for this phenomenon are unknown, high variation may occur in countries where the epidemiology of MRSA is in a transition period (e.g., Germany). Also in countries with a high MRSA proportion, between-hospital variation remains considerable. The large differences between hospitals indicate that initiatives may be most effective when undertaken at the local or regional level (38). To combat the MRSA epidemic, public health researchers and all health professionals must understand the role of hospital hygiene protocols and of antimicrobial drug policies, as well as mechanisms of regional spread of MRSA throughout hospitals. Studies that link information on MRSA guidelines, antimicrobial policies, and prescriptions with resistance rates at the level of the hospital, region, or both, may increase our understanding of the nature of the MRSA epidemic (43).
Acknowledgments
We thank Nico Nagelkerke for his statistical advice, all EARSS participants for delivering their data, the EARSS data managers for entering and checking data and forwarding national data to the central EARSS database, all national representatives for initiating and maintaining the national networks, Stephen Murchan for providing an update on SARI in Ireland, Theresa Lamagni for providing information about the mandatory surveillance of MRSA bacteremia in England, and John Stelling for developing and maintaining the WHONET data-entry program, which is used by many participants.
Dr. Tiemersma works as epidemiologist at the Center for Infectious Diseases Epidemiology at the National Institute for Public Health and the Environment in Bilthoven, the Netherlands. Her research interests focus on the epidemiology of infectious diseases in general and, specifically, on antimicrobial resistance in the Netherlands and in Europe.
This work was performed at the National Institute for Public Health and the Environment, Bilthoven, the Netherlands
References
- Tenover FC, Biddle JW, Lancaster MV. Increasing resistance to vancomycin and other glycopeptides in Staphylococcus aureus. Emerg Infect Dis. 2001;7:327–32. DOIPubMedGoogle Scholar
- World Health Organization. WHO global strategy for containment of antimicrobial resistance. WHO/CDS/CSR/DRS/2001.2. Geneva: The Organization; 2001 [cited 21 Jan 2004]. Available from http://www.who.int/csr/resources/publications/drugresist/EGlobal_Strat.pdf
- Monnet DL. Toward multinational antimicrobial resistance surveillance systems in Europe. Int J Antimicrob Agents. 2000;15:91–101. DOIPubMedGoogle Scholar
- Goettsch W, Bronzwaer SLAM, De Neeling AJ, Wale MC, Aubry-Damon H, Olsson-Liljequist B, Standardization and quality assurance for antimicrobial resistance surveillance of Streptococcus pneumoniae and Staphylococcus aureus within the European Antimicrobial Resistance Surveillance System (EARSS). Clin Microbiol Infect. 2000;6:59–63. DOIPubMedGoogle Scholar
- Bronzwaer SL, Goettsch W, Olsson-Liljequist B, Wale MC, Vatopoulos AC, Sprenger MJ. European Antimicrobial Resistance Surveillance System (EARSS): objectives and organisation. Euro Surveill. 1999;4:41–4.PubMedGoogle Scholar
- Veldhuijzen I, Bronzwaer SL, Degener J, Kool JL. European Antimicrobial Resistance Surveillance System Participants. European Antimicrobial Resistance Surveillance System (EARSS): susceptibility testing of invasive Staphylococcus aureus. Euro Surveill. 2000;5:34–6.PubMedGoogle Scholar
- Tiemersma EW, Lyytikainen O, Bronzwaer S, Schrijnemakers P, Witte W; European Antimicrobial Resistance Surveillance System Participants. Geographical and time trends in proportion of methicillin resistant Staphylococcus aureus (MRSA) in Europe (1999–2001) reported through the European Antimicrobial Resistance Surveillance System (EARSS). In: Abstracts of the 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, California, September 27–30, 2000. Abstract C2-293, 2002. Washington: American Society for Microbiology; 2002.
- Voss A, Milatovic D, Wallrauch-Schwarz C, Rosdahl VT, Braveny I. Methicillin-resistant Staphylococcus aureus in Europe. Eur J Clin Microbiol Infect Dis. 1994;13:50–5. DOIPubMedGoogle Scholar
- Fluit AC, Wielders CL, Verhoef J, Schmitz FJ. Epidemiology and susceptibility of 3,051 Staphylococcus aureus isolates from 25 university hospitals participating in the European SENTRY study. J Clin Microbiol. 2001;39:3727–32. DOIPubMedGoogle Scholar
- Fluit AC, Jones ME, Schmitz FJ, Acar J, Gupta R, Verhoef J. Antimicrobial susceptibility and frequency of occurrence of clinical blood isolates in Europe from the SENTRY antimicrobial surveillance program, 1997 and 1998. Clin Infect Dis. 2000;30:454–60. DOIPubMedGoogle Scholar
- Cookson B. Aspects of the epidemiology of MRSA in Europe. Chemother J. 1995;7(Suppl 3):93–8.PubMedGoogle Scholar
- Grundmann H, Tami A, Hori S, Halwani M, Slack R. Nottingham Staphylococcus aureus population study: prevalence of MRSA among elderly people in the community. BMJ. 2002;324:1365–6. DOIPubMedGoogle Scholar
- Morgan M, Evans-Williams D, Salmon R, Hosein I, Looker DN, Howard A. The population impact of MRSA in a country: the national survey of MRSA in Wales, 1997. J Hosp Infect. 2000;44:227–39. DOIPubMedGoogle Scholar
- Scudeller L, Leoncini O, Boni S, Navarra A, Rezzani A, Verdirosi S, MRSA carriage: the relationship between community and healthcare setting. A study in an Italian hospital. J Hosp Infect. 2000;46:222–9.PubMedGoogle Scholar
- Wertheim HF, Vos MC, Boelens HA, Voss A, Vandenbroucke-Grauls CM, Meester MH, Low prevalence of methicillin-resistant Staphylococcus aureus (MRSA) at hospital admission in the Netherlands: the value of search and destroy and restrictive antibiotic use. J Hosp Infect. 2004;56:321–5. DOIPubMedGoogle Scholar
- Abudu L, Blair I, Fraise A, Cheng KK. Methicillin-resistant Staphylococcus aureus (MRSA): a community-based prevalence survey. Epidemiol Infect. 2001;126:351–6. DOIPubMedGoogle Scholar
- Bronzwaer S, Buchholz U, Courvalin P, Snell J, Cornaglia G, De Neeling A, Comparability of antimicrobial susceptibility test results from 22 European countries and Israel: an external quality assurance exercise of the European Antimicrobial Resistance Surveillance System (EARSS) in collaboration with the United Kingdom National External Quality Assurance Scheme (UK NEQAS). J Antimicrob Chemother. 2002;50:953–64. DOIPubMedGoogle Scholar
- Leegaard TM, Caugant DA, Froholm LO, Hoiby EA. Apparent differences in antimicrobial susceptibility as a consequence of national guidelines. Clin Microbiol Infect. 2000;6:290–3. DOIPubMedGoogle Scholar
- Skov R, Smyth R, Clausen M, Larsen AR, Frimodt-Moller N, Olsson-Liljequist B, Evaluation of a cefoxitin 30 μg disc on Iso-Sensitest agar for detection of methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 2003;52:204–7. DOIPubMedGoogle Scholar
- Austin DJ, Anderson RM. Transmission dynamics of epidemic methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci in England and Wales. J Infect Dis. 1999;179:883–91. DOIPubMedGoogle Scholar
- Monnet DL, Lopez-Lozano JM, Campillos P, Burgos A, Yague A, Gonzalo N. Making sense of antimicrobial use and resistance surveillance data: application of ARIMA and transfer function models. Clin Microbiol Infect. 2001;7:S29–36. DOIPubMedGoogle Scholar
- Kresken M, Hafner D. Drug resistance among clinical isolates of frequently encountered bacterial species in central Europe during 1975–1995. Infection. 1999;27(Suppl 2):S2–8. DOIPubMedGoogle Scholar
- Kresken M, Hafner D, Schmitz FJ, Wichelhaus TA; Study Group Bacterial Resistance of the Paul-Ehrlich-Society for Chemotherapy. Resistenzsituation bei klinisch wichtigen Infektionserregern gegenüber Antibiotika in Deutschland und im mitteleuropäischen Raum. Bericht über die Ergebnisse einer multizentrischen Studie der Arbeitsgemeinschaft Empfindlichkeits-prüfungen & Resistenz der Paul-Ehrlich-Gesellschaft für Chemotherapie e. V. aus dem Jahre 2001. Bonn (Germany): Antiinfectives Intelligence; 2003 [cited 21 Jan 2004]. Available from: http://www.antiinfectives-intelligence.de/peg/ag_resistenz/main.htm
- Speller DC, Johnson AP, James D, Marples RR, Charlett A, George RC. Resistance to methicillin and other antibiotics in isolates of Staphylococcus aureus from blood and cerebrospinal fluid, England and Wales, 1989–95. Lancet. 1997;350:323–5. DOIPubMedGoogle Scholar
- Reacher MH, Shah A, Livermore DM, Wale MC, Graham C, Johnson AP, Bacteraemia and antibiotic resistance of its pathogens reported in England and Wales between 1990 and 1998: trend analysis. BMJ. 2000;320:213–6. DOIPubMedGoogle Scholar
- Birchard K. Ireland tackles antimicrobial resistance in hospitals. Lancet. 2001;357:2113. DOIPubMedGoogle Scholar
- Cunney R. SARI Update. EPI-INSIGHT of the National Disease Surveillance Centre 2002;3:4 [cited 16 Apr 2004]. Available from: http://www.ndsc.ie/Publications/EPI-Insight/2002Issues/d517.pdf
- Health Protection Agency—Communicable Disease Surveillance Centre. The first year of the Department of Health’s mandatory MRSA bacteraemia surveillance scheme in acute NHS Trusts in England: April 2001–March 2002. CDR Weekly. 2002;12:1–17 [cited 21 Apr 2004]. Available from: http://www.hpa.org.uk/cdr/PDFfiles/2002/cdr2502.pdf.
- Department of Health. Mandatory bacteraemia surveillance scheme—MRSA bacteraemia by NHS Trust [cited 21 Apr 2004]. Available from: http://www.dh.gov.uk/assetRoot/04/06/39/46/04063946.pdf.
- Department of Health. Winning ways. Working together to reduce healthcare associated infection in England. Report from the Chief Medical Officer. London: Crown Copyright; 2003 [cited 21 Apr 2004]. Available at http://www.publications.doh.gov.uk/cmo/hai/winningways.pdf.
- Wannet W. Spread of an MRSA clone with heteroresistance to oxacillin in the Netherlands. Euro Surveill. 2002;7:73–4.PubMedGoogle Scholar
- Witte W, Kresken M, Braulke C, Cuny C. Increasing incidence and widespread dissemination of methicillin-resistant Staphylococcus aureus (MRSA) in hospitals in central Europe, with special reference to German hospitals. Clin Microbiol Infect. 1997;3:414–22. DOIPubMedGoogle Scholar
- Melter O, Santos Sanches I, Schindler J, Aires de Sousa M, Mato R, Kovarova V, Methicillin-resistant Staphylococcus aureus clonal types in the Czech Republic. J Clin Microbiol. 1999;37:2798–803.PubMedGoogle Scholar
- Monnet DL, Archibald LK, Phillips L, Tenover FC, McGowan JE Jr, Gaynes RP, Antimicrobial use and resistance in eight US hospitals: complexities of analysis and modeling. Infect Control Hosp Epidemiol. 1998;19:388–94. DOIPubMedGoogle Scholar
- Muto CA, Jernigan JA, Ostrowsky BE, Richet HM, Jarvis WR, Boyce JM, SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and enterococcus. Infection. 2003;24:362–86.PubMedGoogle Scholar
- McDonald P, Mitchell E, Johnson H, Rossney A, Humphreys H, Glynn G, Epidemiology of MRSA: the North/South study of MRSA in Ireland 1999. J Hosp Infect. 2003;54:130–4. DOIPubMedGoogle Scholar
- Kotilainen P, Routamaa M, Peltonen R, Oksi J, Rintala E, Meurman O, Elimination of epidemic methicillin-resistant Staphylococcus aureus from a university hospital and district institutions, Finland. Emerg Infect Dis. 2003;9:169–75.PubMedGoogle Scholar
- Harbarth S, Albrich W, Goldmann DA, Huebner J. Control of multiply resistant cocci: do international comparisons help? Lancet Infect Dis. 2001;1:251–61. DOIPubMedGoogle Scholar
- Archibald L, Phillips L, Monnet D, McGowan JE Jr, Tenover F, Gaynes R. Antimicrobial resistance in isolates from inpatients and outpatients in the United States: increasing importance of the intensive care unit. Clin Infect Dis. 1997;24:211–5.PubMedGoogle Scholar
- Duckworth G. Controlling methicillin resistant Staphylococcus aureus. BMJ. 2003;327:1177–8. DOIPubMedGoogle Scholar
- Duckworth G, Cookson B, Humphreys H, Heathcock R. Revised methicillin-resistant Staphylococcus aureus infection control guidelines for hospitals. Report of a combined working party of the British Society for Antimicrobial Chemotherapy, the Hospital Infection Society and the Infection Control Nurses Association. J Hosp Infect. 1998;39:253–90. DOIPubMedGoogle Scholar
- MacKenzie FM. Inventory of antibiotic resistance and use patterns in European hospitals: first results from ARPAC. Clin Microbiol Infect. 2003;9:S19–130.
Figures
Tables
Cite This Article1European Antimicrobial Resistance Surveillance System national representatives, 2002: Austria: H. Mittermayer, W. Koller; Belgium: H. Goossens, E. Hendrickx; Bulgaria: B. Markova; Croatia: S. Kalenic, A. Tambic-Andrasavic; Czech Republic: P. Urbaskova; Denmark: D. Monnet; Estonia: P. Naaber; Finland: O. Lyytikäinen, A. Nissinen; France: H. Aubry-Damon, P. Courvalin; Germany: U. Buchholz, W. Witte; Greece: N. Legakis, G. Vatopoulos; Hungary: M. Füzi; Ireland: D. O’Flanagan, O. Murphy; Iceland: K. Kristinsson; Israel: R. Raz; Italy: G. Cornaglia, P. D’Ancona; Luxembourg: R. Hemmer; Malta: M. Borg; Netherlands: A. de Neeling, E. Tiemersma; Norway: A. Hoiby, E. Bjørløw; Poland: W. Hryniewicz; Portugal: M. Caniça; Romania: I. Codita; Slovenia: M. Gubina, J. Kolman; Slovakia: L. Langsadl; Spain: F. Baquero, J. Campos; Sweden: B. Liljequist; United Kingdom: A. Johnson, M. Whale.
Table of Contents – Volume 10, Number 9—September 2004
| EID Search Options |
|---|
|
|
|
|
|
|


Please use the form below to submit correspondence to the authors or contact them at the following address:
Edine W. Tiemersma, Center for Infectious Diseases Epidemiology, National Institute for Public Health and the Environment, P.O. Box 1, 3720 BA Bilthoven, the Netherlands; fax: +31-30-274-4409
Top