Volume 11, Number 10—October 2005
Research
Antibacterial Cleaning Products and Drug Resistance
Cite This Article
Citation for Media
Abstract
We examined whether household use of antibacterial cleaning and hygiene products is an emerging risk factor for carriage of antimicrobial drug–resistant bacteria on hands of household members. Households (N = 224) were randomized to use of antibacterial or nonantibacterial cleaning and hygiene products for 1 year. Logistic regression was used to assess the influence of antibacterial product use in homes. Antibacterial product use did not lead to a significant increase in antimicrobial drug resistance after 1 year (odds ratio 1.33, 95% confidence interval 0.74–2.41), nor did it have an effect on bacterial susceptibility to triclosan. However, more extensive and longer term use of triclosan might provide a suitable environment for emergence of resistant species. Further research on this issue is needed.
Concern is growing over the use of household cleaning and hygiene products labeled as antibacterial as a result of laboratory data showing a link between exposure to ingredients in these products, particularly triclosan, and emergence of antimicrobial drug resistance (1–3). This study aimed to determine whether home use of antibacterial cleaning and hygiene products (including use of a handwashing soap containing 0.2% triclosan) or other potential risk factors was associated with carriage of antimicrobial drug–resistant bacteria on household members’ hands. We also assessed the association of these antibacterial products with carriage of organisms with reduced susceptibility to triclosan.
Study Population
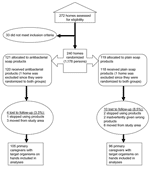
The data for this study were collected as part of a double-masked and randomized home intervention trial (4); participant enrollment began in October 2000, and follow-up occurred for a 12-month period. The methods and randomization procedures for this study have been reported elsewhere (5). A total of 238 households were recruited at baseline; 224 households completed the entire 1-year follow-up (Figure 1). The study was approved by Columbia University Medical Center Institutional Review Board.
Intervention Methods
Households were supplied with over-the counter, generically repackaged consumer cleaning and personal hygiene products free of charge on a monthly or as-needed basis. Households randomly assigned to use antibacterial products received the following: 1) liquid handwashing soap containing 0.2% triclosan, 2) liquid kitchen spray and liquid all-purpose cleaner for hard surfaces that contained a quaternary ammonium component, and 3) oxygenated bleach laundry detergent. Households randomly assigned to the nonantibacterial group received the same products but without antibacterial ingredients. Both groups received the same nonantibacterial liquid dishwashing detergent and bars of body soap to control for potential use of other products that might contain antibacterial ingredients. Study participants were required to use only assigned home hygiene products and were asked not to change any of their normal hygiene practices. Participants, interviewers, and study coordinators were blinded to brand names and ingredients in all products. Adherence to product treatment group was assessed monthly, and products were weighed during each visit to monitor compliance. Households were immediately dropped from the study if they did not adhere to randomized treatments.
Data Collection
At baseline, and quarterly during the 1-year period, a trained interviewer collected demographic information from the person self-identified as the primary caregiver in the household. The baseline interview determined the type of handwashing soap, hygiene, and cleaning products that were used before randomization into the study (i.e., the brand and whether or not the ingredients were labeled as antibacterial). The baseline and quarterly assessment forms provided information such as the number and age of household members, childcare attendance, symptoms of infectious illnesses (fever, diarrhea, sore throat, vomiting, conjunctivitis, skin boils, runny nose), antimicrobial drug use, chronic diseases, self-rated health, birthplace, travel outside of the United States, and occupation. In addition, reported number of handwashes per day by the primary caregiver and a timed observation of the handwash before hand culturing were gathered.
The hands of the primary caregiver were cultured during the home visit at baseline and at the end of the 12-month period before and after washing with the assigned liquid handwashing product. The trained data collector used a coin flip to choose the test hand, which was then inserted into a sterile polyethylene bag containing 50 mL culture medium (0.075 mol/L phosphate buffer, pH 7.9, containing 0.1% polysorbate 80). The hand was massaged for 1 min through the wall of the bag containing culture medium. Only postwash samples were used in analyses since they were considered to be representative of normal versus transient flora found on hands.
Laboratory Methods
The laboratory methods for this study have been described previously (5,6). The microbiologic analysis and antimicrobial drug–susceptibility testing were conducted at New York Presbyterian Hospital, Columbia University Medical Center, New York. Selective media were used to isolate gram-positive cocci, gram-negative bacteria (GNB), Staphylococcus aureus, and enterococci.
Only clinically important bacterial species that were prevalent (species with >38 isolates recovered at baseline and end of year combined) on the hands of homemakers were selected for susceptibility analyses (7,8). These included the following GNB: Acinetobacter baumannii, A. lwoffi, Enterobacter agglomerans, E. cloacae, Klebsiella pneumoniae, and Pseudomonas fluorescens/putida; and the following gram-positive staphylococci: S. aureus, S. warneri, S. epidermidis, and S. capitis. Therefore, only persons who were carrying at least 1 of these organisms on their hands were included in the final analyses (N = 164 at baseline and N = 201 at year-end). No significant differences were noted between the measured demographic characteristics (Tables 1 and 2 for listing of demographics) among those included in the final analyses versus those excluded (all p>0.10).
Bacterial isolates were tested against a panel of antimicrobial agents by using MicroScan WalkAway 96 SI (Dade Behring, Deerfield, IL, USA). Using the recommendations of the Clinical and Laboratory Standards Institute (formerly NCCLS), we classified antimicrobial drug susceptibility as resistant, intermediate, or susceptible to a particular antimicrobial agent (9). Organisms that tested as either resistant or intermediately resistant to antimicrobial agents were classified as "antibiotic resistant" (10). The selection of antimicrobial agents to be tested for each organism was based on clinical applicability of the antimicrobial drug and consistency with earlier studies that examined a link between triclosan and antimicrobial drug resistance (11–14). GNB were tested against several antimicrobial agents, and staphylococci were tested against oxacillin to indicate methicillin resistance. For analytic purposes, GNB species were classified as resistant if a given isolate was resistant to >1 antimicrobial agent(s).
Triclosan susceptibility was examined at Tufts University School of Medicine, Boston, Massachusetts, by using a modified NCCLS agar dilution method (10). Minimum inhibitory concentration (MIC) was defined as the lowest dilution of triclosan that inhibited visible growth. A detailed description of antimicrobial drug and triclosan testing, including controls used and MIC distribution for each organism, has been described previously (6). Since data from the literature regarding triclosan susceptibility testing are sparse and provide no standardized breakpoints (6), we dichotomized triclosan MIC values for each isolate by using the median MIC as a cutoff; low MIC represents less than or equal to the median value and high MIC indicates greater than the median value.
Analytic Methods
First, chi-square and Student t tests were used to compare demographic characteristics of antibacterial and nonantibacterial users. Next, chi-square tests were used to compare the overall proportion of antimicrobial drug–resistant isolates found on the hands of the antibacterial and nonantibacterial groups. Finally, multivariate logistic regression analyses were conducted to examine the relationship between antibacterial product use and 2 separate outcome variables: antimicrobial drug resistance (measured by the presence of >1 antimicrobial drug–resistant species on the hand) and increased triclosan MICs (measured by the presence of >1 species exhibiting a triclosan MIC above the median value).
Each potential covariate (i.e., characteristics of the household and primary caregiver) and our 2 outcome variables were examined in univariate analyses to establish criteria for inclusion in final multivariate models by using a p value <0.05 as the cutoff. Covariates meeting the cutoff criteria were included in multivariate models along with the main effect of the randomized treatment (i.e., antibacterial versus nonantibacterial product use). Analyses were conducted separately for baseline and after 1 year of study participation. Unadjusted and adjusted odds ratios (OR) and 95% confidence intervals (CIs) were generated from logistic regression analyses by using SPSS V.10 (SPSS Inc., Chicago, IL, USA).
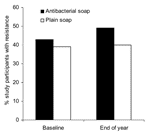
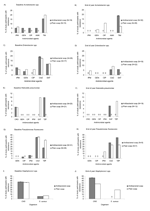
GNB and staphylococci were recovered from 164 participants at baseline and 201 participants at year-end. None of the measured demographic and hygiene characteristics differed significantly between the randomized groups (all p>0.10) (Tables 1 and 2). When comparing isolates from the antibacterial users and nonantibacterial users (Figure 2 and Figure A1), no significant differences in the proportions of resistance were found in all species combined or within single species (all p>0.05).
The odds of carrying >1 antimicrobial drug–resistant strain(s) among antibacterial product users and nonusers were not significant at baseline (OR 0.97, 95% CI 0.50–1.89) or after 1 year of antibacterial product use (OR 1.33, 95% CI 0.74–2.41) (Table 3). In addition, the odds of carrying >1 organism with high triclosan MIC among antibacterial product users or nonusers were similar at baseline (OR 1.59, 95% CI 0.84–3.01) and at year-end (OR 1.73, 95% CI 0.97–3.09).
Individual and Household Characteristics and Susceptibility
At baseline, primary caregivers with higher than average CFU on their hands were twice as likely to carry antimicrobial drug–resistant organisms (Table 3). A slightly increased risk of carrying antimicrobial drug–resistant organisms occurred among those who washed their hands for a longer duration before the culture sample at baseline (Table 3). However, longer duration of handwashing was not associated with reduced bacterial CFU on hands (OR 1.02, 95% CI 0.99–1.06).
At year-end, both the number of times hands were washed per day and the presence of any household member(s) with a healthcare or daycare occupation were significantly associated with reduced carriage of antimicrobial drug–resistant organisms on hands of the primary caregiver (Table 3). Primary caregivers residing in households with members working in healthcare or daycare were significantly more likely to report above-average number of handwashes per day (OR 3.05, 95% CI 1.71–5.44). None of the other characteristics, such as health conditions or antimicrobial drug use, were significantly associated with carriage at baseline or after 1 year (all p>0.05).
This study is the first randomized intervention study to investigate the relationship between antibacterial cleaning and hygiene product use and antimicrobial drug susceptibility of hand microflora within the community setting. Our earlier research, conducted among the same study population described here, showed that use of antibacterial hand soap containing 0.2% triclosan was no more beneficial than plain soap in reducing infectious illness symptoms or bacterial counts on hands of household members (4,5,15). Several avenues of research have contributed to the view that use of products containing triclosan may foster the emergence of antimicrobial drug– or biocide-resistant organisms. This concern stems from reports that exposure to triclosan can lead to bacterial target mutations conferring cross-resistance to isoniazid and selects for mutants bearing resistance to various antimicrobial agents through expression of multidrug-resistant efflux pumps (12,16). Our findings suggest that household use of antibacterial cleaning and hygiene products for a 1-year period is not a significant risk factor for increasing antimicrobial drug–resistant organisms on the hands of persons in the home.
Few data compare resistance patterns among hand microflora and susceptibility to antibacterial handwashing ingredients. One recent cross-sectional study (17) reported a higher prevalence of decreased susceptibility to triclosan among methicillin-resistant S. epidermidis compared to methicillin-sensitive S. epidermidis clinical isolates. The findings reported in other cross-sectional studies have mainly examined environmental and clinical isolates of bacteria, and the correlations reported have been inconsistent (11,13,18–20).
Other Factors Associated with Antimicrobial Drug Resistance
Several hygiene-related factors were significantly associated with carriage, regardless of antibacterial product use. Longer handwashes were slightly associated with increased risk for carriage of antimicrobial drug–resistant species at baseline; as reported previously, these findings may be an artifact of sampling technique (5). The culture was taken directly after the handwash; an increased duration of the wash may have allowed greater dispersal of bacteria into the culture bag.
Primary caregivers residing in households with healthcare or daycare workers had significantly fewer antimicrobial drug–resistant organisms on their hands. This association appears to be influenced by above-average number of handwashes per day by the primary caregiver and indicates that hygiene, regardless of antibacterial ingredients, may reduce household transmission of antimicrobial drug–resistant bacteria.
Limitations for Detecting Changes in Resistance
A factor that might have attenuated the associations found in this study is a higher baseline level of antimicrobial drug resistance in this community. Higher baseline levels would make detecting small changes in susceptibility attributed solely to use of antibacterial cleaning and hygiene products more difficult. Most persons from our study population were from the Dominican Republic, a country that provides over-the-counter access to antimicrobial agents. In an earlier study within this same community, antimicrobial agents were taken by 354 (39%) of 911 persons reporting infectious disease symptoms within the previous 30 days, which suggests high levels of use (21). In addition, this study was conducted for a 1-year period and therefore may not adequately reflect the time-course for development of resistance attributable to use of antibacterial products. Changes in antimicrobial drug resistance during the 1-year period might have been lower than the level of detection that this study was statistically powered to identify. This study was designed to detect an OR >2.11 after 1 year of use, given a power of 80% and a 2-sided α level of 0.05.
Although triclosan susceptibility was examined among various species, we were not able to evaluate potential mechanisms for cross-resistance, such as overexpression of efflux pumps. In addition, when we examined the association between use of antibacterial cleaning and hygiene products and antimicrobial drug resistance, the definition of resistance (>1 organism[s] with antimicrobial drug resistance) did not allow exploration of the potential association with each separate species or antimicrobial drug tested. However, the purpose of our study was to examine overall trends and shifts in antimicrobial drug resistance attributed to the use of antibacterial cleaning and hygiene products, given that the effects of these products in the community are relatively unexplored.
Currently, no evidence suggests that use of antibacterial soap containing 0.2% triclosan provides a benefit over plain soap in reducing bacterial counts and rate of infectious symptoms in generally healthy persons in the household setting (4,5,15). Our 1-year randomized community intervention study adds to these earlier findings by assessing the potential risks associated with antibacterial product use in the home. The results from our study do not implicate use of antibacterial cleaning and hygiene products as an influential factor in carriage of antimicrobial drug–resistant bacteria on the hands of household members. Although we did not observe a significant impact on antimicrobial drug resistance during the 1-year period, a longer duration and more extensive use of triclosan might provide a suitable environment for emergence of antimicrobial drug–resistant species in the community setting. Further surveillance for the effect of long-term use of antibacterial cleaning and hygiene products on antimicrobial drug resistance in the community is needed.
At the time of this writing, Dr Aiello was a Robert Wood Johnson Health & Society Scholar in the Department of Epidemiology, Center for Social Epidemiology & Population Health, at the University of Michigan School of Public Health. She is now an Assistant Professor of Epidemiology at that center. Her research interests include antimicrobial drug and antibacterial resistance within the community and clinical setting, multidisciplinary approaches for examining antimicrobial drug resistance, and life-course socioeconomic determinants of infectious diseases.
Acknowledgments
This study was funded by Home Hygiene Practices and Infection Transmission in Households, 3 R01, NR05251-02s1, funded by National Institute of Nursing Research, National Institutes of Health. Support was also given by the Robert Wood Johnson Health & Society Scholars Program. The Center for Infectious Disease and Epidemiological Research provided a National Institute of Allergy and Infectious Diseases training fellowship at Columbia University.
A.E. Aiello conceived the aims of the study, conducted the statistical analyses, and wrote the manuscript; B. Marshall, S. B. Levy, and P. Della-Latta conducted the clinical testing of the samples and contributed to the concepts and writing of the manuscript; S. Lin aided in the data management and provided statistical consultation and review of the manuscript; and E. Larson created the home hygiene study design and contributed to the concepts and writing of the manuscript.
References
- Aiello AE, Larson E. Antibacterial cleaning and hygiene products as an emerging risk factor for antimicrobial drug resistance in the community. Lancet Infect Dis. 2003;3:501–6. DOIPubMedGoogle Scholar
- Levy SB. Antibiotic and antiseptic resistance: impact on public health. Pediatr Infect Dis J. 2000;19(Suppl):S120–2. DOIPubMedGoogle Scholar
- Levy SB. Antibacterial household products: cause for concern. Emerg Infect Dis. 2001;7(Suppl):512–5.PubMedGoogle Scholar
- Larson E, Lin SX, Gomez-Duarte C, Della-Latta P. Effect of antibacterial home cleaning and handwashing products on infectious disease symptoms. Ann Intern Med. 2004;140:321–9.PubMedGoogle Scholar
- Larson E, Aiello A, Lee LV, Della-Latta P, Gomez-Duarte C, Lin S. Short- and long-term effects of handwashing with antimicrobial or plain soap in the community. J Community Health. 2003;28:139–50. DOIPubMedGoogle Scholar
- Aiello AE, Marshall B, Levy SB, Della-Latta P, Larson E. Relationship between triclosan and susceptibilities of bacteria isolated from hands in the community. Antimicrob Agents Chemother. 2004;48:2973–9. DOIPubMedGoogle Scholar
- NNIS. National Nosocomial Infections Surveillance (NNIS) System report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32:470–85. DOIPubMedGoogle Scholar
- Wisplinghoff H, Edmond MB, Pfaller MA, Jones RN, Wenzel RP, Seifert H. Nosocomial bloodstream infections caused by Acinetobacter species in United States hospitals: clinical features, molecular epidemiology, and antimicrobial susceptibility. Clin Infect Dis. 2000;31:690–7. DOIPubMedGoogle Scholar
- National Committee for Clinical Laboratory Standards, ed. Performance standards for antimicrobial disk susceptibility tests M2-A6. Wayne (PA): The Committee; 1997.
- National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard- 6th edition. Wayne (PA): The Committee; 2003.
- Suller MT, Russell AD. Triclosan and antibiotic resistance in Staphylococcus aureus. J Antimicrob Chemother. 2000;46:11–8. DOIPubMedGoogle Scholar
- Schweizer HP. Triclosan: a widely used biocide and its link to antimicrobial agents. FEMS Microbiol Lett. 2001;202:1–7. DOIPubMedGoogle Scholar
- Cole EC, Addison RM, Rubino JR, Leese KE, Dulaney PD, Newell MS, Investigation of antibiotic and antibacterial agent cross-resistance in target bacteria from homes of antibacterial product users and nonusers. J Appl Microbiol. 2003;95:664–76. DOIPubMedGoogle Scholar
- Chuanchuen R, Beinlich K, Hoang TT, Becher A, Karkhoff-Schweizer RR, Schweizer HP. Cross-resistance between triclosan and antimicrobial agents in Pseudomonas aeruginosa is mediated by multidrug efflux pumps: exposure of a susceptible mutant strain to triclosan selects nfxB mutants overexpressing MexCD-OprJ. Antimicrob Agents Chemother. 2001;45:428–32. DOIPubMedGoogle Scholar
- Aiello AE, Lee LV, Della-Latta P, Lin S, Larson E. Types of microbial flora on hands of subjects and an environmental surface in homes randomized to antimicrobial or non-antimicrobial cleaning and hygiene products. In: IDSA, editor. 40th annual meeting of the infectious disease society of America. Chicago (IL): Infectious Disease Society of America; 2002.
- Chuanchuen R, Karkhoff-Schweizer RR, Schweizer HP. High-level triclosan resistance in Pseudomonas aeruginosa is solely a result of efflux. Am J Infect Control. 2003;31:124–7. DOIPubMedGoogle Scholar
- Schmid MB, Kaplan N. Reduced triclosan susceptibility in methicillin-resistant Staphylococcus epidermidis. Antimicrob Agents Chemother. 2004;48:1397–9. DOIPubMedGoogle Scholar
- Cookson BD, Farrelly H, Stapleton P, Garvey RP, Price MR. Transferable resistance to triclosan in MRSA. Lancet. 1991;337:1548–9. DOIPubMedGoogle Scholar
- Bamber AI, Neal TJ. An assessment of triclosan susceptibility in methicillin-resistant and methicillin-sensitive Staphylococcus aureus. J Hosp Infect. 1999;41:107–9. DOIPubMedGoogle Scholar
- Lear JC, Maillard JY, Dettmar PW, Goddard PA, Russell AD. Chloroxylenol- and triclosan-tolerant bacteria from industrial sources. J Ind Microbiol Biotechnol. 2002;29:238–42. DOIPubMedGoogle Scholar
- Larson E, Lin SX, Gomez-Duarte C. Antibiotic use in Hispanic households, New York City. Emerg Infect Dis. 2003;9:1096–102.PubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 11, Number 10—October 2005
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Allison E. Aiello, Robert Wood Johnson Health and Society Scholar, Center for Social Epidemiology & Population Health, University of Michigan, School of Public Health, 1214 S. University, Ann Arbor, MI 48104-2548; fax: 734-998-0006
Top