Volume 11, Number 7—July 2005
Dispatch
Household Transmission of Gastroenteritis
Cite This Article
Citation for Media
Abstract
Transmission of infectious gastroenteritis was studied in 936 predominately Hispanic households in northern California. Among 3,916 contacts of 1,099 primary case-patients, the secondary attack rate was 8.8% (95% confidence interval 7.9–9.7); children had a 2- to 8-fold greater risk than adults. Bed-sharing among children in crowded homes is a potentially modifiable risk.
Infectious diarrhea poses a major problem for the US healthcare system and employers. Although <5% of episodes may result in a physician encounter (1), surveillance systems have estimated that 0.72 episodes occur per person-year, and up to 1.1 episodes per year for children <5 years of age (2). Household transmission of infectious gastroenteritis is likely to account for a substantial portion of community incidence. With the exception of a few prospective studies (3,4), studies of household transmission of gastroenteritis have typically reported on community outbreaks of individual pathogens followed up in the home (5–9). In these outbreak settings, secondary attack rates were 4%–20%, depending on pathogen, mode of transmission, and length of time spent in the household. Since household clusters of gastroenteritis may parallel larger community trends (10), information about baseline incidence and risk factors is useful to validate population-based and sentinel surveillance systems.
Index cases of probable infectious gastroenteritis were identified through 15 participating community health providers; 11 of these were public health clinics serving low-income families. After an initial telephone interview, a home visit was scheduled within 2 weeks of the index episode. Household contacts of the index patient were interviewed regarding symptoms and onset of diarrheal illness. A second visit 3 months later completed documentation of the household episode. The cohort consists predominately of Hispanic families with young children, including a median of 5 persons (range 2–20) per household and a median sleeping density of 2.5 persons/bedroom. Households were excluded if they had <2 participating members or if the living unit was a dormitory or other communal residential arrangement.
Infectious gastroenteritis was defined as 1) diarrheal illness lasting <14 days and marked by symptoms of loose or watery stool occurring at least 5 times per day in a child <2 years of age, or at least 3 times per day in older persons or 2) at least 1 instance of vomiting per day in a person of any age. Illnesses considered noninfectious in origin, such as those due to morning sickness, poisoning, medications, or alcohol, were excluded from the case definition. A primary case was defined as the first household case with onset within 2 weeks of the index referral or onset within 2 days of the first primary case. Secondary gastroenteritis was defined as an illness meeting the case definition of infectious gastroenteritis, beginning at least 2 days after the onset and ≤5 days after the end of an episode in another household contact. A household episode was deemed to have concluded when all members had been symptom-free for at least 5 days. Secondary attack rates were estimated crudely and also modeled by using the life-table method. Risk factors associated with secondary transmission were assessed with logistic regression, with an exchangeable correlation matrix to account for household clusters.
From 1999 to 2004, a total of 3,747 index referrals were received; 2,094 (56%) persons could be contacted by phone and met initial eligibility criteria. Of these, 830 (40%) declined participation, and 1,264 (60%) were scheduled for a home visit. After the initial home visit, 1,154 households were enrolled, and 102 were excluded because the index episode did not meet the case definition (n = 80), the household had <2 interested members (n = 20), or someone in the household had participated in a prior study (n = 2). Of the 1,154 households enrolled, 936 (81%) completed documentation of ≥1 household gastroenteritis episode. These 936 households had 5,783 members. Of these, 5,015 (87%) gave reports sufficient to classify symptoms as primary, secondary, or absent; 557 (9.6%) did not participate in interviews; and 211 (3.6%) reported diarrhea, vomiting, or both that could not be classified temporally. Of participating members, 24% were ≤5 years of age, 18% were 6–17 years of age, and 58% were ≥18 years of age.
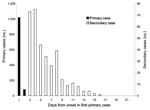
Household episodes lasted a median of 9 days (range 5–29 days) and involved 1–6 household members. Of the 1,443 (29%) household members who reported symptoms consistent with infectious gastroenteritis, 1,099 (76%) were classified as primary and 344 (24%) as secondary case-patients (Figure 1). Median age of primary cases was 3.6 years, including 60% <5 years of age (Table 1).
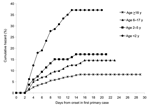
Among 3,916 contacts of the 1,099 primary case-patients, the crude secondary attack rate was 8.8% (95% confidence interval [CI] 7.9–9.7). Household transmission occurred within a median of 4 days (range 2–15 days) of onset of symptoms in the first primary case. Cumulative hazard rates varied substantially by age: 37%, 17%, 15%, and 8% for study participants <2, 2–5, 6–17, and ≥18 years of age, respectively (Figure 2). Secondary transmission occurred in 240 (26%) homes, and households with secondary cases were larger, with a median of 3 versus 2 children.
The crude secondary attack rate of 9% is somewhat lower than, but not inconsistent with, prior estimates. Pickering et al. (5) reported an overall secondary attack rate of 11% among family members of children involved in daycare outbreaks, with rates of 26%, 15%, and 17% for Shigella, rotavirus, and Giardia, respectively. After a foodborne outbreak of Norovirus, Gotz et al. (6) estimated that 20% of family members of daycare participants became ill. In an investigation of sporadic Escherichia coli O157, Parry et al. (9) estimated attack rates of 4%–15% in household contacts. In a small Danish community, 12% of family members became ill after children attending a daycare center in a neighboring town were exposed to a contaminated water supply (11). Similarly, 11% of family members became ill within 3 days of children's return from summer camp, where they were exposed to a suspected viral agent (8).
Since outbreak and surveillance investigations typically focus on highly transmissible agents with more severe illnesses, the somewhat lower secondary attack rate observed in this study of unidentified, mixed agents is not surprising. Risk interval (ours including a 96-hour postsymptomatic period) may affect classification of secondary illness. Among 835 households completing the follow-up visit, 380 (46%) had up to 7 recurrent episodes during a 3-month period, 42 beginning 6–10 days after conclusion of the first episode. Merging these episodes would have increased crude attack rates slightly, to ≈10%. Conversely, >80% of secondary cases occurred within 7 days of primary onset. Thus, our risk interval was likely to capture incubation periods of more common causal agents in the United States, although the fact that these homes were susceptible to recurrent episodes over time cannot be ignored.
The risk factor analysis (Table 2) confirmed the role of age in household transmission; children exhibited a 2- to 8-fold greater risk for secondary gastroenteritis compared with adults. Although 60% of patients with primary cases were <5 years of age, secondary transmission was more likely when the primary case-patient was >5 years (adjusted odds ratio [AOR] 1.7 [95% CI 1.3–2.3]). In addition, being a member of the index family (AOR 2.5 [95% CI 1.7–3.6]) and sharing a bed with a primary case-patient (AOR 2.0 [95% CI 1.5–2.6]) were independently associated with risk. As observed in some studies of Norovirus (6,12), exposure to vomiting was also associated with household transmission (AOR 1.6 [95% CI 1.2–2.2]), despite the fact that only 1 of 5 contacts reported this exposure.
Viral agents are thought to account for 80% of reported community diarrhea (13), and repeated exposure to agents like rotavirus, as is likely to occur in homes with small children, may be associated with features of acquired immunity (14). Some support for this hypothesis is the fact that, compared with primary cases, secondary cases tended to be in older children, with shorter episodes and fewer vomiting episodes (Table 1). Conversely, children <5 years of age, who constituted nearly one fourth of household members and had more than half of all illnesses, had more protracted episodes, regardless of primary or secondary onset or symptoms. Thus, although age-related immunity may have played a role in modifying duration or severity of secondary illness, the high proportion of young children was more determinative of household risk. Bed-sharing with these children was likely the major factor in propagating infection in these crowded homes.
Although our enrollment rate is similar to that in other population-based studies of gastroenteritis (1,15), we cannot exclude the possibility that participating families had different attack rates than nonparticipants. Approximately 13% of members in participating households were not interviewed or could not be classified as to temporal onset of symptoms; however, ascertainment did not differ between households with and without secondary cases. Although 19% of households were lost to follow-up, most commonly because of relocation, attack rates among completing households available for 1 visit or 2 were not significantly different. Our risk factor model, which assumes that multiple events within households share a common correlation across time, may be strong for longitudinal data, although for more limited risk periods and conditions of intense, close contact, the approach may be plausible.
In conclusion, household transmission continues to play an important role in community rates of acute intestinal infections. Bed-sharing among children in crowded homes constitutes a potentially modifiable risk.
Dr. Perry is an epidemiologist with the Parsonnet Laboratory in the Division of Geographic Medicine and Infectious Diseases at Stanford University School of Medicine. Her research interests include immunoepidemiology of Helicobacter pylori, Mycobacterium tuberculosis, and intestinal co-infection.
Acknowledgments
The authors gratefully acknowledge the assistance of the following healthcare providers: Santa Clara Valley Medical Center Emergency Department, Santa Clara Valley Medical Center Pediatrics Clinic, Santa Clara Valley Medical Center East Valley Pediatric and Urgent Care Clinics, Santa Clara County Office of Environmental Health, Mayview Community Health Center, Mayview Community Health Center, San Mateo County General Hospital Pediatric and Primary Care Clinics, Stanford Hospital Emergency Department, and Willow Clinic.
This work is supported by National Institutes of Health grant AI42801.
References
- Wheeler JG, Sethi D, Cowden JM, Wall PG, Rodrigues LC, Tompkins DS, Study of infectious intestinal disease in England: rates in the community, presenting to general practice, and reported to national surveillance. The Infectious Intestinal Disease Study Executive. BMJ. 1999;318:1046–50. DOIPubMedGoogle Scholar
- Imhoff B, Morse D, Shiferaw B, Hawkins M, Vugia D, Lance-Parker S, Burden of self-reported acute diarrheal illness in FoodNet surveillance areas, 1998–1999. Clin Infect Dis. 2004;38(Suppl 3):S219–26. DOIPubMedGoogle Scholar
- Dingle J, Badger G, Jordan WJ. Illness in the home: a study of 251,000 illnesses in a group of Cleveland families. Cleveland (OH): Case Western Reserve University Press; 1964.
- Koopman JS, Monto AS, Longini IM Jr. The Tecumseh study. XVI: Family and community sources of rotavirus infection. Am J Epidemiol. 1989;130:760–8.PubMedGoogle Scholar
- Pickering LK, Evans DG, DuPont HL, Vollet JJ III, Evans DJ Jr. Diarrhea caused by Shigella, rotavirus, and Giardia in day-care centers: prospective study. J Pediatr. 1981;99:51–6. DOIPubMedGoogle Scholar
- Gotz H, de JB, Lindback J, Parment PA, Hedlund KO, Torven M, et al. Epidemiological investigation of a food-borne gastroenteritis outbreak caused by Norwalk-like virus in 30 day-care centres. Scand J Infect Dis. 2002;34:115–21. DOIPubMedGoogle Scholar
- Kaplan JE, Schonberger LB, Varano G, Jackman N, Bied J, Gary GW. An outbreak of acute nonbacterial gastroenteritis in a nursing home. Demonstration of person-to-person transmission by temporal clustering of cases. Am J Epidemiol. 1982;116:940–8.PubMedGoogle Scholar
- Morens DM, Zweighaft RM, Vernon TM, Gary GW, Eslien JJ, Wood BT, A waterborne outbreak of gastroenteritis with secondary person-to-person spread. Association with a viral agent. Lancet. 1979;1:964–6. DOIPubMedGoogle Scholar
- Parry SM, Salmon RL. Sporadic STEC O157 infection: secondary household transmission in Wales. Emerg Infect Dis. 1998;4:657–61. DOIPubMedGoogle Scholar
- Ethelberg S, Olsen KE, Gerner-Smidt P, Mølbak K. Household outbreaks among culture-confirmed cases of bacterial gastrointestinal disease. Am J Epidemiol. 2004;159:406–12. DOIPubMedGoogle Scholar
- Laursen E, Mygind O, Rasmussen B, Ronne T. Gastroenteritis: a waterborne outbreak affecting 1600 people in a small Danish town. J Epidemiol Community Health. 1994;48:453–8. DOIPubMedGoogle Scholar
- Heun EM, Vogt RL, Hudson PJ, Parren S, Gary GW. Risk factors for secondary transmission in households after a common-source outbreak of Norwalk gastroenteritis. Am J Epidemiol. 1987;126:1181–6.PubMedGoogle Scholar
- Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, Food-related illness and death in the United States. Emerg Infect Dis. 1999;5:607–25. DOIPubMedGoogle Scholar
- Velazquez FR, Matson DO, Calva JJ, Guerrero L, Morrow AL, Carter-Campbell S, Rotavirus infections in infants as protection against subsequent infections. N Engl J Med. 1996;335:1022–8. DOIPubMedGoogle Scholar
- Hoogenboom-Verdegaal AM, de Jong JC, During M, Hoogenveen R, Hoekstra JA. Community-based study of the incidence of gastrointestinal diseases in The Netherlands. Epidemiol Infect. 1994;112:481–7. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 11, Number 7—July 2005
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Sharon Perry, Stanford University School of Medicine, 300 Pasteur Dr, HRP (Redwood) Bldg, Room T225, Stanford, CA 94305, USA; fax: 650-725-6951
Top