Volume 11, Number 7—July 2005
Dispatch
Enterotoxigenic Escherichia coli and Vibrio cholerae Diarrhea, Bangladesh, 2004
Cite This Article
Citation for Media
Abstract
Flooding in Dhaka in July 2004 caused epidemics of diarrhea. Enterotoxigenic Escherichia coli (ETEC) was almost as prevalent as Vibrio cholerae O1 in diarrheal stools. ETEC that produced heat-stable enterotoxin alone was most prevalent, and 78% of strains had colonization factors. Like V. cholerae O1, ETEC can cause epidemic diarrhea.
In July 2004, Bangladesh experienced devastating floods, which also affected the capital, Dhaka, and outbreaks of diarrheal diseases occurred throughout the city. As a result, a steep increase was seen in patient admissions, which reached epidemic numbers around July 20, when >350 patients were admitted every day to the hospital of the International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B). During the peak period, >700 patients were seen per day, and the total number seen during the epidemic was >17,000.
Diarrhea caused by enterotoxigenic Escherichia coli (ETEC) is highly prevalent in young children in developing countries as well as in travelers to these areas (1). In Bangladesh, Vibrio cholerae is the bacterial pathogen that most frequently necessitates hospitalization (2). ETEC is also commonly isolated from patients seeking treatment in hospitals (3–5), but it is not actively screened for during natural disasters. However, reports have suggested that ETEC, in addition to cholera, is a predominant cause of diarrhea in Bangladesh (6,7). Since ETEC spreads though contaminated water and food (8,9), we analyzed diarrheal stools for this pathogen to assess the prevalence of ETEC during the epidemic.
ETEC causes diarrhea by producing different combinations of the heat labile (LT) or heat stable (ST) enterotoxins and 1 or more of at least 22 different colonization factors, which contribute to the virulence of the pathogen (10). Since genes for these factors are predominantly present on plasmids, which may be lost on storage, we tested for phenotypic expression of these factors by using freshly cultured isolates. For this purpose, diarrheal stools were collected from patients in a 2% systematic routine surveillance system; every 50th patient attending the hospital is routinely screened for V. cholerae, Shigella spp., and Salmonella spp (4). at the Clinical Research and Service Centre of the ICDDR, B. The study was approved by the institutional review board of ICDDR,B.
Only samples negative for V. cholerae were tested for ETEC, starting from July 20, 2004, when the patient numbers increased at the ICDDR,B hospital for ≈6 weeks, until the patient numbers decreased and the floods had receded. Information, including age, sex, fever, vomiting, dehydration status, and related clinical features, was also collected from patients. For ETEC surveillance, we used lactose-fermenting E. coli colonies cultured on MacConkey agar plates that had been cultured from fresh stool specimens (4). Six lactose-fermenting individual colonies of E. coli were tested for the presence of LT, ST, and colonization factors. Detection of LT and ST was carried out with ganglioside GM1 enzyme-linked immunosorbent assays (4). The colonies that tested positive for the toxins were also plated onto colonization factor antigen (CFA) agar plates with and without bile salts for testing colonization factors (4). Trypticase soy agar containing 5% sheep blood (TSA) was used to test for the colonization factor CS21 (5).
The strains were cultured at 37°C overnight; those grown on CFA agar without bile were tested for colonization factors CFA/1, CSI, CS2, CS3, CS4, and CS6, and those on CFA agar plus bile were tested for CS5, CS7, CS17, CS8, CS12, and CS14 (4). Those strains grown on TSA were tested for CS21 only (5). Of the patients included in this study, 67% had severe-to-moderate dehydration; of these, 51% were children <5 years of age, while 39% were >15 years of age. They were treated for diarrhea with oral (61%) or intravenous (39%) rehydration therapy and other medications as needed.
Of 350 stool specimens tested during the epidemic, 78 (22.2%) were positive for V. cholerae O1 (22 Ogawa and 56 Inaba serotype), and 63 (18.0%) were positive for ETEC. Shigella spp. (3.4%, n = 11) and Salmonella spp. (1.7%, n = 5) were seen at lower rates. Children with ETEC diarrhea were negative for V. cholerae O1 as well as Shigella spp. and Salmonella spp. We did not test V. cholerae–positive samples for ETEC and therefore cannot rule out possible concomitant infection with ETEC in these 78 cholera patients (4).
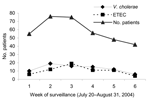
Isolation of ETEC and V. cholerae O1 remained high throughout the epidemic (Figure), and during 1 week, comparable numbers of ETEC and V. cholerae were isolated from stools of patients. We compared demographic and clinical features of patients with ETEC and V. cholerae infections (Table 1). Most patients with ETEC diarrhea were <2 years of age (56%) or >15 years of age (36%) (median 1.5 years), whereas those with V. cholerae O1 infection were mostly >5 years of age (median age 15.5 years). Although more cholera patients had severe dehydration (60%), 22% of the patients with ETEC diarrhea also had severe dehydration (p<0.001). Intravenous rehydration was needed for both ETEC- and V. cholerae–infected patients, but it was more frequently used in the latter.
With regard to toxin profile, ETEC expressing ST alone was the most common (67%), followed by strains producing both ST and LT (19%) and LT alone (14%). Dominance of the ST-expressing ETEC has been documented earlier during seasonal outbreaks and epidemics in Bangladesh (4) and in Egypt and the Middle East (11,12). Patients infected with the different toxin phenotypes of ETEC had dehydration status ranging from severe to none, although no significant association was seen between toxin phenotype and degree of dehydration.
A high proportion of the ETEC strains (78%) expressed 1 or more colonization factors (Table 2), a much higher frequency than that seen in other hospital or community-based studies (10,12). In earlier studies in Bangladesh, we found 56% of strains positive for these colonization factors (4). In the present study, ≈92% of ST/LT-, 79% of ST-, and 56% of LT-expressing ETEC expressed 1 or more colonization factors. CFA/I was the most common phenotype, followed by the strains expressing CS4 + CS6 or CS5 + CS6, followed by others. Thus, most of the colonization factor types were those known to be present in clinical strains and those that have previously been isolated from hospitalized patients (4,5). These antigens have been given priority for designing vaccines to protect against a wide range of colonization factors (10). In addition, 3 strains co-expressed CS21, a type IV pilus antigen (4). Of these, 2 strains expressed CFA/I and CS21, and 1 was positive for CS1, CS3, and CS21.
We used 13 colonization factor–specific monoclonal antibodies in testing; however, >22 colonization factors have been described, not all of which could be tested in this study. In addition, although precautions were taken to rule out the loss of phenotypic properties of colonization factors, some may have been lost on culture. By using polymerase chain reaction or DNA hybridization procedures, more colonization factor–specific genes and those that have undergone phenotypic changes could have been detected (13).
We hypothesize that contaminated water during floods can be a cause of ETEC diarrhea. Flood waters may be contaminated by sewage, increasing transmission by the fecal-oral route. Our recent studies have also shown that ETEC can be isolated relatively frequently from surface water samples in Bangladesh (14).
Although diarrhea can be prevented by improving water quality, sanitation, and overall hygiene, these improvements will not be possible in the near future in densely populated areas with limited resources. Thus, developing vaccines that can prevent such epidemics is a goal. Such vaccines should include at least the most prevalent colonization factors, such as those found on the ETEC strains we isolated, to provide protection against the virulent, colonization factor–expressing, ST-positive ETEC strains.
This article emphasizes that ETEC can be a major source of acute watery diarrhea in epidemics caused by floods. This report is the first to show that during waterborne natural disasters, ETEC can also cause dehydrating diarrhea severe enough to require clinical care and, in many instances, intravenous rehydration. During epidemics, focus on ETEC should be on pediatric patients <2 years of age, since ETEC was the most prevalent bacterial enteropathogen identified in this age group. The treatment strategy should be designed accordingly, since ETEC strains are becoming increasingly resistant to erythromycin (15), the drug usually used for young children with acute watery diarrhea, irrespective of diagnosis.
Dr. Qadri is senior scientist and head of the Immunology Unit, ICDDR,B. Her major research interests are studies of enteric pathogens, especially enterotoxigenic Escherichia coli and Vibrio cholerae, with emphasis on immunologic and epidemiologic studies of natural infections and vaccine development.
Acknowledgment
This work was supported by the ICDDR,B Centre for Health and Population Research. We acknowledge with gratitude the Swedish Agency for Research and Economic Cooperation (Sida-SAREC, grant no. 2001-3970) and National Institutes of Allergy and Infectious Diseases (grant UO1 AI58935) to the Centre's research efforts.
References
- World Health Organization. New frontiers in the development of vaccines against enterotoxigenic (ETEC) and enterohaemorrhagic (EHEC) E. coli infections. Part 1. Wkly Epidemiol Rec. 1999;74:98–101.PubMedGoogle Scholar
- Sack RB, Siddique AK, Longini IM, Nizam A Jr, Yunus M, Islam MS, A 4-year study of the epidemiology of Vibrio cholerae in four rural areas of Bangladesh. J Infect Dis. 2003;187:96–101. DOIPubMedGoogle Scholar
- Black RE, Merson MH, Rowe B, Taylor PR, Abdul Alim AR, Gross RJ, Enterotoxigenic Escherichia coli diarrhoea: acquired immunity and transmission in an endemic area. Bull World Health Organ. 1981;59:263–8.PubMedGoogle Scholar
- Qadri F, Das SK, Faruque AS, Fuchs GJ, Albert MJ, Sack RB, Prevalence of toxin types and colonization factors in enterotoxigenic Escherichia coli isolated during a 2-year period from diarrheal patients in Bangladesh. J Clin Microbiol. 2000;38:27–31.PubMedGoogle Scholar
- Qadri F, Giron JA, Helander A, Begum YA, Asaduzzaman M, Xicohtencatl-Cortes J, Human antibody response to longus type IV pilus and study of its prevalence among enterotoxigenic Escherichia coli in Bangladesh by using monoclonal antibodies. J Infect Dis. 2000;181:2071–4. DOIPubMedGoogle Scholar
- Faruque AS, Salam MA, Faruque SM, Fuchs GJ. Aetiological, clinical and epidemiological characteristics of a seasonal peak of diarrhoea in Dhaka, Bangladesh. Scand J Infect Dis. 1998;30:393–6. DOIPubMedGoogle Scholar
- Khan MU, Eeckels R, Alam AN, Rahman N. Cholera, rotavirus and ETEC diarrhoea: some clinico-epidemiological features. Trans R Soc Trop Med Hyg. 1988;82:485–8. DOIPubMedGoogle Scholar
- Ohno A, Marui A, Castro ES, Reyes AA, Elio-Calvo D, Kasitani H, Enteropathogenic bacteria in the La Paz River of Bolivia. Am J Trop Med Hyg. 1997;57:438–44.PubMedGoogle Scholar
- Olsvik O, Wasteson Y, Lund A, Hornes E. Pathogenic Escherichia coli found in food. Int J Food Microbiol. 1991;12:103–13. DOIPubMedGoogle Scholar
- Gaastra W, Svennerholm AM. Colonization factors of human enterotoxigenic Escherichia coli (ETEC). Trends Microbiol. 1996;4:444–52. DOIPubMedGoogle Scholar
- Rao MR, Abu-Elyazeed R, Savarino SJ, Naficy AB, Wierzba TF, Abdel-Messih I, High disease burden of diarrhea due to enterotoxigenic Escherichia coli among rural Egyptian infants and young children. J Clin Microbiol. 2003;41:4862–4. DOIPubMedGoogle Scholar
- Wolf MK. Occurrence, distribution, and associations of O and H serogroups, colonization factor antigens, and toxins of enterotoxigenic Escherichia coli. Clin Microbiol Rev. 1997;10:569–84.PubMedGoogle Scholar
- Steinsland H, Valentiner-Branth P, Grewal HM, Gaastra W, Mølbak KK, Sommerfelt H. Development and evaluation of genotypic assays for the detection and characterization of enterotoxigenic Escherichia coli. Diagn Microbiol Infect Dis. 2003;45:97–105. DOIPubMedGoogle Scholar
- Begum YA, Talukder KA, Nair GB, Svennerholm AM, Sack RB, Qadri F. Enterotoxigenic Escherichia coli isolated from surface water in urban and rural Bangladesh. J Clin Microbiol. 2005. In press. DOIPubMedGoogle Scholar
- Chakraborty S, Deokule JS, Garg P, Bhattacharya SK, Nandy RK, Nair GB, Concomitant infection of enterotoxigenic Escherichia coli in an outbreak of cholera caused by Vibrio cholerae O1 and O139 in Ahmedabad, India. J Clin Microbiol. 2001;39:3241–6. DOIPubMedGoogle Scholar
Figure
Tables
Cite This ArticleTable of Contents – Volume 11, Number 7—July 2005
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Firdausi Qadri, Laboratory Sciences Division, ICDDR,B, GPO Box 128, Dhaka 1000, Bangladesh; fax: 880-2-8802-8823116
Top