Volume 12, Number 3—March 2006
Research
West Nile Virus Infections Projected from Blood Donor Screening Data, United States, 2003
Figure 3
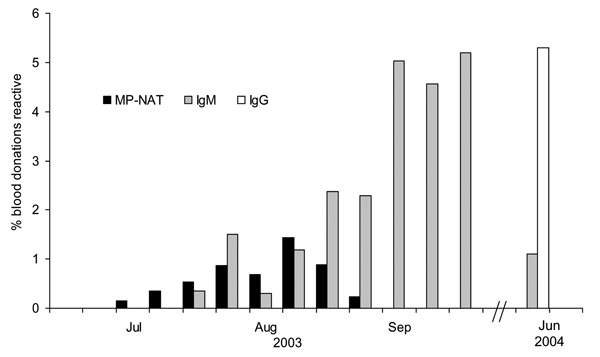
Figure 3. West Nile virus minipool–nucleic acid amplification testing (MP-NAT) yield and immunoglobulin M (IgM) and IgG seroprevalence estimates for North Dakota, during and ≈8 months after the 2003 epidemic period.
Page created: January 27, 2012
Page updated: January 27, 2012
Page reviewed: January 27, 2012
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.