Volume 12, Number 5—May 2006
Letter
Postmortem Confirmation of Human Rabies Source
To the Editor: Rabies is a fatal encephalitis caused by a neurotropic RNA virus of the family Rhabdoviridae, genus Lyssavirus. The predominant rabies virus reservoir hosts are bats and carnivores. Among these, rabid dogs represent a substantial public health problem, particularly in developing countries (1).
Laboratory diagnosis of rabies is essential to guide control programs, epidemiologic surveys, and prophylactic measures (2). Among the laboratory tests recommended by the World Health Organization (WHO), the fluorescent antibody test (FAT) is the accepted standard for rabies diagnosis (1). Although rabies virus antigens can be detected in decomposed samples, FAT is less effective when such samples are tested. In those cases, polymerase chain reaction (PCR) can provide better results (3). Since the degree of decomposition at which FAT starts to become ineffective is unknown (4), when smears from decomposed samples are made for FAT, a suspension of the same brain tissues should be made in the appropriate diluents for the mouse inoculation test (MIT), cell culture, or reverse transcription–polymerase chain reaction (RT-PCR) (2). However, if all test results are negative, rabies cannot be ruled out because of the condition of the sample.
On February 28, in the city of Carbonita, Minas Gerais State, in southeastern Brazil, a 62-year-old man was bitten by a bat on the right ankle. Approximately 50 days later, his leg began to feel numb, and he experienced a continuous headache, pain at the site of the bite, convulsions, frequent urge to clear his throat, hiccups, nausea, difficulty in swallowing, dry lips, slightly elevated body temperature (37°C–37.5°C), paralysis of superior and inferior left limbs, shaking, and hallucinations. On May 4, 16 days after clinical manifestations began, the patient died; the cause of death was registered as a cerebral vascular accident. One month later, the body was exhumed to obtain a sample from the central nervous system (CNS), which was sent to Instituto Pasteur, São Paulo, registered as sample 5341 M/04 and tested by FAT, MIT, and RT-PCR.
In total, 8 smears were prepared from the sample to be analyzed by FAT according to the method of Dean et al. (5) with fluorescein isothiocyanate–labeled polyclonal antinucleocapsid antibodies. MIT was carried out as described by Koprowski (6) with 7 mice. For RT-PCR, RNA was extracted from the CNS sample with TRIzol, according to the manufacturer's instructions (Invitrogen, Rockville, MD, USA). RT-PCR was carried out with modifications as described by Orciari et al. (7), with primers 504 (sense) and 304 (antisense), aiming at the amplification of a 249-bp fragment of rabies virus nucleoprotein (N) gene, by using Superscript II (Invitrogen) and Taq DNA-polymerase (Invitrogen).
Fluorescent inclusions were observed in 6 of the 8 slides prepared for the FAT. The RT-PCR of the RNA sample resulted in amplicons of the correct size (249 bp), as did the positive control sample, CVS strain rabies virus. No bands were observed in the reaction corresponding to the negative/reagent control (ultra-pure water). The MIT results were negative. Because the virus could not be isolated, antigenic typing with monoclonal antibodies could not be performed.
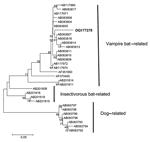
The fragment obtained in the RT-PCR was bidirectionally sequenced with DYEnamic ET Dye Terminator (Amersham Biosciences, Piscataway, NJ, USA) in a MegaBACE DNA sequencer (Amersham Biosciences) and resulted in a 165-nucleotide sequence. The final sequence was aligned with homologous sequences from GenBank by using the ClustalW (available from http://www.ebi.ac.uk/clustalw) and Bioedit software (Isis Pharmaceuticals, Carlsbad, CA, USA). The phylogenetic tree was produced by using the neighbor-joining DNA-distance method and the Kimura 2-parameter model with 1,000 bootstrap replicates in Mega 2.1 (version 2.1) (available from http://www.megasoftware.net/). The sequence was segregated in the variant 3 cluster (Desmodus rotundus–related variants), which suggests that D. rotundus is the most probable source of infection (Figure). The sequence was assigned GenBank accession no. DQ177278.
The lack of diagnosis or delay in diagnosis can increase the number of persons potentially exposed to rabies virus infection by contact with the patient or even by organ transplantations (8). Moreover, an early diagnosis can decrease the cost of treatment by eliminating the use of ineffective drugs and unnecessary diagnostic tests (2), as well as allowing potentially useful emerging therapeutic strategies to be used (9).
Before this report, no reference of a rabies diagnosis by FAT or RT-PCR had been reported from a human exhumed 30 days postmortem. The RT-PCR results agree with those obtained by David et al. (10) from a decomposed sample of animal origin after 36 days.
These facts demonstrate that rabies should be considered in cases of encephalitis with the classic clinical signs and symptoms as well as the paralytic form of disease (paresis and paralysis). Rabies should be suspected when early clinical symptoms, for example, itching and paresthesia, are demonstrated at the local site of infection. In addition, the laboratory investigation showed that molecular methods such as RT-PCR and sequencing were sensitive assays for nucleic acid detection and determination of the rabies virus variant in this unusual case from an exhumed human.
References
- Rupprecht CE, Halon CA, Hemachudha T. Rabies re-examined. Lancet Infect Dis. 2002;2:327–43. DOIPubMedGoogle Scholar
- Trimarchi CV, Smith JS. Diagnostic evaluation. In: Jackson AC, Wunner WH, editors. Rabies. San Diego: Academic Press; 2002. p. 307–49.
- Soares RM, Bernardi F, Sakamoto SM, Heinemann MB, Cortez A, Alves LM, A heminested polymerase chain reaction for the detection of Brazilian rabies isolates from vampires bats and herbivores. Mem Inst Oswaldo Cruz. 2002;97:109–11. DOIPubMedGoogle Scholar
- Albas A, Ferrari CIL, Da Silva LHQ, Bernardi F, Ito FH. Influence of canine brain decomposition on laboratory diagnosis of rabies. Rev Soc Bras Med Trop. 1999;32:19–22. DOIPubMedGoogle Scholar
- Dean DJ, Abelseth MK, Atanasiu P. The fluorescent antibody test. In: Meslin FX, Kaplan MM, Koprowski H, editors. Laboratory techniques in rabies. Geneva: World Health Organization; 1996. p. 88–95.
- Koprowski H. The mouse inoculation test. In: Meslin FX, Kaplan MM, Koprowski H, editors. Laboratory techniques in rabies. Geneva: World Health Organization; 1996. p. 80–7.
- Orciari LA, Niezgoda M, Hanlon CA, Shaddock JH, Sanderlin DW, Yager PA, Rapid clearance of SAG-2 rabies virus from dogs after oral vaccination. Vaccine. 2001;19:4511–8. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Investigation of rabies infections in organ donor and transplant recipients—Alabama, Arkansas, Oklahoma, and Texas, 2004. MMWR Morb Mortal Wkly Rep. 2004;53:586–9.
- Willoughby RE Jr, Tieves KS, Hoffman GM, Ghanayem NS, Amlie-Lefond CM, Schwabe MJ, Survival after treatment of rabies with induction of coma. N Engl J Med. 2005;352:2508–14. DOIPubMedGoogle Scholar
- David D, Yakobson B, Rotenberg D, Dveres N, Davidson I, Stram Y. Rabies virus detection by RT-PCR in decomposed naturally infected brains. Vet Microbiol. 2002;87:111–8. DOIPubMedGoogle Scholar
Figure
Cite This ArticleRelated Links
Table of Contents – Volume 12, Number 5—May 2006
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Rafael Oliveira, Instituto Pasteur, Laboratory of Diagnosis in Virology, Av Paulista, 393 Cerqueira Cesar São Paulo, São Paulo 01311-001, Brazil
Top