Volume 12, Number 8—August 2006
Research
Venezuelan Equine Encephalitis Virus Transmission and Effect on Pathogenesis
Cite This Article
Citation for Media
Abstract
Quantifying the dose of an arbovirus transmitted by mosquitoes is essential for designing pathogenesis studies simulating natural infection of vertebrates. Titration of saliva collected in vitro from infected mosquitoes may not accurately estimate titers transmitted during blood feeding, and infection by needle injection may affect vertebrate pathogenesis. We compared the amount of Venezuelan equine encephalitis virus collected from the saliva of Aedes taeniorhynchus to the amount injected into a mouse during blood feeding. Less virus was transmitted by mosquitoes in vivo (geometric mean 11 PFU) than was found for comparable times of salivation in vitro (mean saliva titer 74 PFU). We also observed slightly lower early and late viremia titers in mice that were needle injected with 8 PFU, which represents the low end of the in vivo transmission range. No differences in survival were detected, regardless of the dose or infection route.
Designing pathogenesis studies for arboviruses that accurately simulate natural infection requires quantifying the amount of virus transmitted. Virus assays on mosquito saliva can be used to estimate the amount transmitted to vertebrates during blood feeding. However, amount of virus collected in vitro may not accurately reflect mosquito transmission.
Indirect and direct methods can be used to quantify virus delivered in mosquito saliva. Indirect methods include comparing times of death of animals exposed to a mosquito bite with those infected parenterally with known doses and comparing the time between mosquito feeding and development of viremia with the time between needle injection and development of the same viremia level. Direct methods include quantifying virus salivated into drops of blood, virus detected in vertebrate tissues immediately after mosquito feeding, virus salivated into blood agar, and virus salivated into fluids such as oil. Saliva collection in oil-filled capillary tubes, first described by Hurlbut (1), is widely used. Chamberlain et al. (2) compared several indirect and direct methods for quantifying arbovirus transmission and concluded that allowing mosquitoes to feed on serum (similar to the capillary method) is less efficient in detecting virus than other methods. Since most saliva is expectorated during probing, salivation into hanging drops or capillary tubes may be inaccurate because mosquitoes do not need to salivate to locate a blood vessel.
The amount of several arboviruses transmitted by mosquitoes has been estimated by using artificial saliva collection (1–12). Up to 3 log10 PFU of eastern equine encephalitis virus is deposited into capillary tubes filled with oil by the vector Culiseta melanura (9). Capillary collection and real-time reverse transcription (RT)–PCR estimate that Culex pipiens pipiens saliva contains an average of 4.3 log10 PFU of West Nile virus (WNV), with a range of 0.5 to 5.3 log10 (8). Recently, we estimated that the epidemic Venezuelan equine encephalitis virus (VEEV) vector, Aedes (Ochlerotatus) taeniorhynchus, salivates 0.2–3.2 log10 PFU into oil-filled capillary tubes (12).
Vector saliva enhances infection with many pathogens (13–18), and mosquito saliva is reported to enhance infection by some arboviruses. Deer and chipmunks infected with La Crosse virus by the bite of Ae. (Och.) triseriatus have higher and longer viremias than animals infected by needle (19). Mice exposed to uninfected mosquitoes and injected at the feeding site with Cache Valley virus develop enhanced viremia and seroconversion compared with unbitten mice or to those co-injected with virus and mosquito saliva (20). Mice have higher seroconversion rates to vesicular stomatitis virus when infected by Ae. triseriatus than by needle injection (21). Cytokine expression in the skin of mice infected with Sindbis virus differs after injection with mosquito salivary gland extracts than after injection with virus alone (22).
Other studies report no enhancement of arbovirus infection by vector saliva or feeding. Hamsters infected with WNV by mosquitoes or needle injections do not differ in level or duration of viremia, clinical manifestations, pathology, or antibody response (23). Birds infected with western equine encephalitis virus or Saint Louis encephalitis virus by mosquito bite or needle exhibit no difference in viremia responses (24), and mosquito saliva inhibits in vitro infection of dendritic cells by dengue virus (25).
VEEV (family Togaviridae, genus Alphavirus) is an important emerging and reemerging pathogen of humans and equines in the neotropics. Since no effective antiviral agents or a licensed human vaccine for VEEV exists, therapy is primarily supportive and prevention relies on avoidance of mosquitoes. Outbreaks of VEE can involve hundreds of thousands of equine and human cases, spread over large regions, and can last several years (26).
The effect of vector feeding on vertebrate infections by VEEV has not been studied. We determined the amount of VEEV in mosquito saliva collected in vitro (12) but did not determine whether this amount accurately reflects transmission during blood feeding. To collect saliva in a capillary tube, we need to subject the mosquito to traumatic manipulations that may affect salivation, such as anesthetization or immobilization by removal of the legs and wings. Also, mosquitoes are usually allowed to salivate into capillary tubes for a longer time (e.g., 30 minutes) than is required for engorgement on a host. Because knowing the infectious dose transmitted by mosquitoes is important for designing vertebrate infection studies, in which needles are typically used for virus delivery, we compared the amount of VEEV transmitted by mosquitoes in vitro with that transmitted in vivo. We also determined whether mosquito transmission affects viremia or time to death when compared with needle infections. Finally, we used tail amputations to investigate the extravascular or intravascular location of VEEV deposition during mosquito feeding.
Virus
We used VEEV rescued from an infectious cDNA clone derived from epidemic strain 3908 (subtype IC), a 1995 human isolate from Zulia State, Venezuela (27). With the exception of some IE virus strains in Mexico, subtype IC viruses are the etiologic agents of all recent VEE epidemics. Strain 3908 was passaged once in C6/36 mosquito cells before isolation of viral RNA and production of infectious cDNA. Virus recovered from baby hamster kidney cells electroporated with transcribed RNA was used for all experiments. Use of virus derived from an infectious clone minimized attenuating mutations that occur when VEEV is passaged in cell culture (28).
Mosquitoes
Ae. taeniorhynchus F1 progeny of mosquitoes captured in Florida (29) were reared at 27°C and a relative humidity of 80% in a light:dark cycle of 12:12 hours. Adult females were infected intrathoracically with 4 log10 PFU of VEEV in a 1-μL volume 6–8 days after emergence and incubated at 27°C for 5 days with 10% sucrose provided ad libitum. Intrathoracic infection of mosquitoes with VEEV and incubation for 5 days generates saliva titers comparable to those that occur after oral infection (12).
In Vivo Transmission
Thirty-nine 6- to 8-week-old National Institutes of Health (NIH) Swiss mice (Harlan, Indianapolis, IN, USA) were anesthetized with pentobarbital, and the distal portion of the tail was exposed to 1 infected mosquito. After mosquito engorgement, the tips of the tails of 29 mice were severed and immediately homogenized in 300 μL of Eagle's minimal essential medium (MEM) supplemented with 20% fetal bovine serum (FBS) in a Mixer Mill 300 (Retsch Inc., Newton, PA, USA); the tails of 10 control mice were left intact. After centrifugation at 9,000×g for 5 minutes, the supernatant was removed for cell culture assays and RNA extraction with a Qiagen kit (Qiagen, Valencia, CA, USA). Vero cells were injected with 30 μL of supernatant and observed for 5 days for cytopathic effects (CPEs). All CPE-positive samples were titrated by plaque assay on Vero cells.
RNA was also extracted from the pellet of the tail homogenate with Trizol (Invitrogen, Carlsbad, CA, USA). The RNA of both supernatant and tail pellet was tested for VEEV positive-strand RNA by using real-time RT-PCR with a one-step kit (Qiagen) and a Smart Cycler (Cepheid, Sunnyvale, CA, USA). Forward (5´-CATAGTCTAGTCCGCCAAGATGTT-3´) and reverse (5´-CGATAGGGCATTGGCTGCAT-3´) primers and a probe (5´-[6-FAM]CCCGTTCCAACCAATGTAT[NFQ-MGB]-3´) were used for amplification and detection, respectively. The assay consisted of reverse transcription at 50°C for 20 minutes, denaturation at 95°C for 10 minutes, and 45 cycles at 95°C for 15 seconds, 63°C for 30 seconds, and 72°C for 30 seconds. Virus titers were extrapolated from RT-PCR results by comparison with a standard curve generated from serial dilutions of a VEEV stock quantified by plaque assay.
After a mosquito probed or fed on the mouse tail, its infection was confirmed by using forced salivation into a capillary tube as described below. All 39 mice used were kept in individual cages, monitored for signs of infection, and bled retroorbitally 2 weeks later to test for seroconversion by using plaque-reduction neutralization tests.
To ensure that all virus injected into mouse tails could be recovered and detected, a series of known doses (2.9 log10, 2 log10, and 1 log10 PFU) was injected intradermally into the tail of a mouse. The tips were then severed and processed as described above. Each dose was tested in duplicate, and nearly all the injected virus was recovered (mean 2.7 log10 PFU recovered for 2.9 log10 inoculum, mean 1.9 log10 PFU recovered for 2.0 log10 inoculum, and mean 0.8 log10 PFU recovered for 1.0 log10 inoculum). Samples with known virus titers were also tested to ensure that freezing and thawing once did not alter virus content.
Saliva Assays
Thirty-nine saliva samples from intrathoracically infected mosquitoes that fed on a mouse were obtained by immobilization (legs and wings removed) and forced salivation into 10-μL capillary tubes (VWR International, West Chester, PA, USA) containing immersion oil (type B, Cargille Laboratories Inc., Cedar Grove, NJ, USA). After 30–45 minutes, salivation was confirmed by appearance of bubbles at the tip of the proboscis. An additional cohort of mosquitoes was allowed to salivate for intervals (repeated in triplicate) to duplicate times of observed mosquito feeding. The oil-saliva mixture was centrifuged into 100 μL of MEM containing 20% FBS and frozen at -80°C; 30 μL was then added to Vero cells for detection of CPE. Mosquito infection was confirmed by assaying triturated bodies and legs and wings for CPE, followed by plaque assay.
Viremia and Death
Ten 6- to 8-week-old NIH Swiss mice were infected by either 1 mosquito or intradermal injection into the ear with either 0.9 or 3.4 log10 PFU, which represented the range of titers injected by mosquitoes (see below). Five mice from each cohort of 10 were bled retroorbitally at 12, 24, 36, 48, 72, 96, and 120 hours postinfection, and sera were titrated by plaque assay. Mice were monitored daily until signs of encephalitis appeared, after which they were observed 4 times a day to determine time of death. The University of Texas Medical Branch Institutional Animal Care and Use Committee approved all experiments.
Statistical Analysis
Log-transformed data were normally distributed, except for data from RT-PCR assays from mouse tail homogenate pellets on which 1 mosquito probed (this group was not compared statistically). One-way analysis of variance using Tukey's test for multiple comparisons and an unpaired t test were used to compare all normally distributed data with GraphPad Prism 4.0 (GraphPad Software, San Diego, CA, USA).
In Vivo Versus In Vitro Transmission Titers
To determine whether saliva collection accurately approximates the amount of VEEV transmitted during a mosquito bloodmeal, we quantified virus from saliva collected in vitro and virus deposited at sites of in vivo blood feeding. One mosquito feeding on the distal portion of a mouse tail transmitted a mean ± standard deviation of 1.1 ± 1.0 log10 PFU (11 PFU) as detected in the homogenate supernatant and 0.8 ± 0.9 log10 PFU (7 PFU) as estimated by real-time RT-PCR. These amounts were significantly lower (p<0.001) than the mean ± standard deviation amount (3.6 ± 1.5 log10 PFU or 4,300 PFU) deposited into capillary tubes during 30–45 minutes of salivation (Figure 1). However, the time for engorgement (<3 minutes) was much shorter than the 45 minutes allowed for in vitro salivation. Therefore, we matched times of saliva collection (range <3 minutes) to the exact engorgement times. Less VEEV (p<0.05) was still detected after in vivo transmission than after <3 minutes of in vitro salivation (1.9 ± 1.2 log10 PFU or 74 PFU).
The effect that the time of probing or feeding had on the titer of virus salivated was analyzed by timed saliva collections and mouse tail exposures. The amount of VEEV collected from mosquitoes that salivated in vitro for <3 minutes was significantly less than the amount collected from mosquitoes allowed to salivate for 45 minutes (p<0.0001). However, no significant difference was seen in the amount transmitted by mosquitoes allowed to completely engorge compared with mosquitoes allowed to probe only, without engorgement (p>0.05, 95% confidence interval -0.8 to 1.5 log10 PFU for the difference in the mean titers).
To address the possibility that some virus injected by feeding mosquitoes rapidly binds to or penetrates mouse cells and therefore is not measured by plaque assay, we also examined VEEV RNA content in mouse tails. No difference was detected between mean virus content in the mouse tail homogenate supernatants assayed by RT-PCR or plaques (Figure 1). Detection of relatively small amounts of viral RNA in tail homogenate pellets indicated that almost all virus remained in the supernatant and that infectious virus was not underestimated because of rapid penetration of cells or binding of virus to connective tissue (Figure 1).
Location of VEEV Deposition In Vivo
To assess intravascular versus extravascular locations of VEEV deposition by mosquitoes, we amputated the distal portions of mouse tails immediately after engorgement, and mice were observed for signs of infection. Forty percent (4/10) of control mice whose tails were not amputated after mosquito feeding survived compared with 79% (23/29) of those whose tails were amputated (p = 0.04, by Fisher exact test). No mice that survived developed neutralizing antibodies. NIH Swiss mice infected with VEEV have a death rate approaching 100%, which indicates that a systemic VEEV infection did not occur in surviving animals. This suggested that nearly all saliva and associated virus were deposited extravascularly and confined to the bite site by a lack of immediate vascular dissemination. Tail amputation nearly doubled survival rates by removing this virus before replication and dissemination.
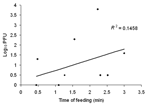
Virus Transmitted Versus Time of Engorgement
To assess the temporal pattern of virus deposition during blood feeding, the amount of VEEV transmitted was compared with the time required for mosquito engorgement. Figure 2 shows no correlation between feeding time and amount of VEEV in mouse tails, suggesting that most virus in saliva was deposited early during probing, with minimal deposition during engorgement.
Effect on Pathogenesis in Mice Infected by Needle Versus Mosquito
To determine whether mosquito saliva affects pathogenesis of VEEV infection, mice were infected by either the bite of 1 mosquito or by intradermal needle injection. Two VEEV doses were used to represent the range of titers injected during blood feeding (Figure 1). Viremia from needle injection with a high dose did not differ from that generated by mosquito transmission (Figure 3). In contrast, viremia from a mosquito bite was higher than that from a needle injection of a low dose for the 12-hour (p<0.05) and 96-hour (p<0.001) time points. A significant difference in viremia (p<0.001) was also observed at the 12-hour and 96-hour time points for mice infected by needle injection of a high dose than infection of a low dose. No difference was detected in the mean survival times of mice infected by either mosquito (5.9 ± 0.6 days) or needle injection with 8 PFU (6.4 ± 0.7days) or 3.4 log10 PFU (6.3 ± 0.4 days) (Figure 4).
Studies with some (19–21) but not all (23,24) arboviruses suggest that natural infection by mosquito bite may potentiate arboviral infection compared with parenteral infection. Because the effect of mosquito transmission on infection by VEEV has not been addressed, we assessed the infectious dose delivered by a natural vector, Ae. taeniorhynchus, compared with estimated doses from saliva collections. We also evaluated the effect of mosquito VEEV transmission on pathogenesis in mice.
In Vivo Versus In Vitro Transmission
Our results indicate that Ae. taeniorhynchus transmit less VEEV in vivo than they deposit into a capillary tube, even when saliva collection times are matched to engorgement times. We therefore caution against extended times of saliva collection in capillary tubes because mosquitoes allowed to salivate for 45 minutes expel more VEEV than those that salivate for <3 minutes, the approximate maximum time required for natural engorgement.
Our study also assessed the location of saliva deposition. As reasoned by Turell et al. (30,31), if an arbovirus were deposited intravascularly, it would quickly circulate beyond the bite site and animals with tail amputations would still become infected and die. Turell et al. reported that when the tails of suckling mice are exposed to a VEEV-infected Ae. taeniorhynchus and the tails are amputated £10 minutes later, 31%–37% survive compared with 4% of mice whose tails were not amputated (30). Our results indicating that the odds of dying are decreased by ≈50% for those whose tails were amputated suggest that saliva and VEEV are deposited both intravascularly and extravascularly. This conclusion is slightly different than that of Turell et al. (30,31), who concluded that mosquitoes inject most virus extravascularly and only small amounts intravascularly or that intravascular transmission occurs only occasionally. An explanation for the differences in death rates found in our studies and those of Turell et al. is that they used suckling mice, whereas we used adult mice. Two of our mice that had been only probed by an infected mosquito also became infected. Surprisingly, no VEEV was detected in the tail homogenate of these mice by either cell culture or RT-PCR. Because the 50% mouse subcutaneous lethal dose (LD50) for VEEV strain 3908 administered in the tail is 12 PFU (D.R. Smith, unpub. data), which is greater than the LD50 for injection in the thigh (12) and an amount detectable by our methods, virus was probably deposited primarily intravascularly in these 2 animals.
Forty percent of our mice with intact tails survived after allowing an infected mosquito to engorge. NIH Swiss mice are highly susceptible to VEEV; death rates are typically 100%. Therefore, our results and those from our previous study (12), which reported that infected mosquitoes often deposit <12 PFU of VEEV into capillary tubes, suggest that systemically infected Ae. taeniorhynchus frequently transmit little or no virus. In contrast to the 40% survival rate of mice with intact tails, 100% of mice infected by mosquito bite at another site died, presumably because of a difference in the site of virus deposition. The subcutaneous LD50 for VEEV strain 3908 administered in the tail is 12 PFU compared with <1 PFU in the leg (D.R. Smith, unpub. data). Mosquitoes may deposit different amounts of virus at different anatomic sites, depending on accessibility of blood vessels.
Time of Engorgement and Infectious Dose Transmitted
The amount of VEEV transmitted by Ae. taeniorhynchus did not correlate with time of engorgement. However, we did not count how many times the mosquito probed before beginning to engorge. Assuming that most mosquito saliva is injected during the intradermal probing period that precedes cannulation of a blood vessel and that infection of the host correlates with the duration of salivation during probing, probing frequency could affect transmission outcome and should be investigated.
Effects on VEE Pathogenesis of Infection by Needle Versus Mosquito
Because mosquitoes transmit a wide range of arbovirus doses, we injected mice with 2 doses that represented the range of VEEV transmitted in vivo. No difference in viremia was detected between mice infected by a mosquito than by needle injection of a high dose. However, mice infected by mosquito bite showed higher viremia titers at the early (12 hours) and late (96 hours) time points than did mice infected with a low dose given by needle. Because mice injected with the high dose also had higher viremia titers at some time points than did mice in the low-dose cohort (Figure 3), the difference in the infection by mosquito bite versus a low-dose injection by needle may indicate only that some mosquitoes transmitted doses >8 PFU. The only way to confirm this slight effect of mosquito transmission on early and late viremia would be to duplicate the exact distribution of in vivo transmission titers by needle injections. However, volumes injected by mosquitoes compared with those injected by needles would differ, as would intradermal sites of deposition. Another approach is to co-inject mosquito saliva with virus (20,22), but the same volume and site discrepancies would apply.
Although route (mosquito versus needle) or dose of VEEV had no detectable effect on death rates (Figure 4), mosquito transmission enhancement of early viremia titers could affect subsequent vector infection, and comparable studies with natural reservoir or amplification hosts are needed to assess this possibility. In preliminary studies, no difference in the viremia response of spiny rats was observed after VEEV infection by needle compared with mosquito bites (A.S. Carrara and S.C. Weaver, unpub. data).
Our results agree with reports of little or no enhancement of alphaviral infections by mosquito transmission (24). In several studies describing enhancement of arboviral infection by mosquito transmission, multiple mosquitoes were allowed to feed and transmit to 1 vertebrate (19–21), or salivary gland extracts from many mosquitoes were injected with virus (20,22). Because natural infection rates of mosquitoes are typically low, simultaneous transmission by >1 vector is probably rare. In addition, virus amounts injected by needle in these studies may have been less than the virus amount transmitted by mosquitoes, which would confound interpretation. Artificial conditions used for several experiments demonstrating potentiation of arbovirus infection by mosquito transmission may therefore exaggerate the true effect.
Significance for Pathogenesis Studies
In conclusion, Ae. taeniorhynchus transmit less VEEV in vivo than they deposit in vitro into capillary tubes. Mosquito transmission of VEEV has little or no effect on the overall murine viremia profile and none on death. To design VEE pathogenesis studies that simulate natural infection, a dose range from 10 PFU to 1,000 PFU is recommended to simulate mosquitoborne infections. Because VEEV saliva titers differ among mosquito species (12), comparable studies should be conducted with other vectors.
Ms Smith is a graduate student at the University of Texas Medical Branch in Galveston. Her research interests include mechanisms of arbovirus transmission by mosquitoes and the pathogenesis of VEEV in both the mosquito vectors and vertebrate hosts.
Acknowledgments
We thank Charles Fulhorst for statistical advice, Nikos Vasilakis and Joseph Masterson for technical advice, and Jing Huang for rearing mosquitoes.
Darci R. Smith was supported by Centers for Disease Control and Prevention training grant T01/CCT622892. Patricia V. Aguilar and Lark L. Coffey were supported by the James W. McLaughlin Fellowship Fund. This research was supported by National Institutes of Health grants AI 418807 and AI 57156.
References
- Hurlbut HS. Mosquito salivation and virus transmission. Am J Trop Med Hyg. 1966;15:989–93.PubMedGoogle Scholar
- Chamberlain RW, Kissling RE, Sikes RK. Studies on the North American arthropod-borne encephalitides. VII. Estimation of amount of eastern equine encephalitis virus inoculated by infected Aedes aegypti. Am J Hyg. 1954;60:286–91.PubMedGoogle Scholar
- Aitken TH. An in vitro feeding technique for artificially demonstrating virus transmission by mosquitoes. Mosq News. 1977;37:130–3.
- Collins WE. Transmission of Semliki Forest virus by Anopheles albimanus using membrane feeding techniques. Mosq News. 1963;23:96–9.
- Gubler DJ, Rosen L. A simple technique for demonstrating transmission of dengue virus by mosquitoes without the use of vertebrate hosts. Am J Trop Med Hyg. 1976;25:146–50.PubMedGoogle Scholar
- Mellink JJ. Transmission of Venezuelan equine encephalomyelitis virus by Aedes aegypti (Diptera: Culicidae) to mice previously exposed to vector antigens. J Med Entomol. 1982;19:371–5.PubMedGoogle Scholar
- Ross RW. A laboratory technique for studying the insect transmission of animal viruses, employing a bat-wing membrane, demonstrated with two African viruses. J Hyg (Lond). 1956;54:192–200. DOIPubMedGoogle Scholar
- Vanlandingham DL, Schneider BS, Klingler K, Fair J, Beasley D, Huang J, Real-time reverse transcriptase-polymerase chain reaction quantification of West Nile virus transmitted by Culex pipiens quinquefasciatus. Am J Trop Med Hyg. 2004;71:120–3.PubMedGoogle Scholar
- Weaver SC, Scott TW, Lorenz LH. Patterns of eastern equine encephalomyelitis virus infection in Culiseta melanura (Diptera: Culicidae). J Med Entomol. 1990;27:878–91.PubMedGoogle Scholar
- Ross RW. Transmission experiments with chikungunya A and B virus. Experiments using a new apparatus. Entebbe, Uganda: Virus Research Institute; 1953. p. 13–14.
- Davis NC. Attempts to determine the amount of yellow fever virus injected by the bite of a single infected Stegomyia mosquito. Am J Trop Med. 1934;14:343–54.
- Smith DR, Carrara A, Aguilar P, Weaver SC. Evaluation of methods to assess transmission potential of Venezuelan equine encephalitis virus by mosquitoes and estimation of mosquito saliva titers. Am J Trop Med Hyg. 2005;73:33–9.PubMedGoogle Scholar
- Titus RG, Ribeiro JM. Salivary gland lysates from the sand fly Lutzomyia longipalpis enhance Leishmania infectivity. Science. 1988;239:1306–8. DOIPubMedGoogle Scholar
- Shaw MK, Tilney LG, McKeever DJ. Tick salivary gland extract and interleukin-2 stimulation enhance susceptibility of lymphocytes to infection by Theileria parva sporozoites. Infect Immun. 1993;61:1486–95.PubMedGoogle Scholar
- Ribeiro JM. Role of saliva in blood-feeding by arthropods. Annu Rev Entomol. 1987;32:463–78. DOIPubMedGoogle Scholar
- Nuttall PA, Jones LD, Labuda M, Kaufman WR. Adaptations of arboviruses to ticks. J Med Entomol. 1994;31:1–9.PubMedGoogle Scholar
- Titus RG, Ribeiro JM. The role of vector saliva in transmission of arthropod-borne disease. Parasitol Today. 1990;6:157–60. DOIPubMedGoogle Scholar
- Theodos CM, Titus RG. Salivary gland material from the sand fly Lutzomyia longipalpis has an inhibitory effect on macrophage function in vitro. Parasite Immunol. 1993;15:481–7. DOIPubMedGoogle Scholar
- Osorio JE, Godsey MS, Defoliart GR, Yuill TM. La Crosse viremias in white-tailed deer and chipmunks exposed by injection or mosquito bite. Am J Trop Med Hyg. 1996;54:338–42.PubMedGoogle Scholar
- Edwards JF, Higgs S, Beaty BJ. Mosquito feeding-induced enhancement of Cache Valley virus (Bunyaviridae) infection in mice. J Med Entomol. 1998;35:261–5.PubMedGoogle Scholar
- Limesand KH, Higgs S, Pearson LD, Beaty BJ. Potentiation of vesicular stomatitis New Jersey virus infection in mice by mosquito saliva. Parasite Immunol. 2000;22:461–7. DOIPubMedGoogle Scholar
- Schneider BS, Soong L, Zeidner NS, Higgs S. Aedes aegypti salivary gland extracts modulate anti-viral and TH1/TH2 cytokine responses to Sindbis virus infection. Viral Immunol. 2004;17:565–73. DOIPubMedGoogle Scholar
- Sbrana E, Tonry JH, Xiao SY, da Rosa AP, Higgs S, Tesh RB. Oral transmission of West Nile virus in a hamster model. Am J Trop Med Hyg. 2005;72:325–9.PubMedGoogle Scholar
- Reisen WK, Chiles RE, Kramer LD, Martinez VM, Eldridge BF. Method of infection does not alter response of chicks and house finches to western equine encephalomyelitis and St. Louis encephalitis viruses. J Med Entomol. 2000;37:250–8. DOIPubMedGoogle Scholar
- Ader DB, Celluzzi C, Bisbing J, Gilmore L, Gunther V, Peachman KK, Modulation of dengue virus infection of dendritic cells by Aedes aegypti saliva. Viral Immunol. 2004;17:252–65. DOIPubMedGoogle Scholar
- Weaver SC, Ferro C, Barrera R, Boshell J, Navarro JC. Venezuelan equine encephalitis. Annu Rev Entomol. 2004;49:141–74. DOIPubMedGoogle Scholar
- Weaver SC, Salas R, Rico-Hesse R, Ludwig GV, Oberste MS, Boshell J, Re-emergence of epidemic Venezuelan equine encephalomyelitis in South America. VEE Study Group. Lancet. 1996;348:436–40. DOIPubMedGoogle Scholar
- Bernard KA, Klimstra WB, Johnston RE. Mutations in the E2 glycoprotein of Venezuelan equine encephalitis virus confer heparan sulfate interaction, low morbidity, and rapid clearance from blood of mice. Virology. 2000;276:93–103. DOIPubMedGoogle Scholar
- Coffey LL, Weaver SC. Susceptibility of Ochlerotatus taeniorhynchus and Culex nigripalpus for Everglades virus. Am J Trop Med Hyg. 2005;73:11–6.PubMedGoogle Scholar
- Turell MJ, Spielman A. Nonvascular delivery of Rift Valley fever virus by infected mosquitoes. Am J Trop Med Hyg. 1992;47:190–4.PubMedGoogle Scholar
- Turell MJ, Tammariello RF, Spielman A. Nonvascular delivery of St. Louis encephalitis and Venezuelan equine encephalitis viruses by infected mosquitoes (Diptera: Culicidae) feeding on a vertebrate host. J Med Entomol. 1995;32:563–8.PubMedGoogle Scholar
Figures
Cite This ArticleTable of Contents – Volume 12, Number 8—August 2006
| EID Search Options |
|---|
|
|
|
|
|
|


Please use the form below to submit correspondence to the authors or contact them at the following address:
Scott C. Weaver, Department of Pathology, University of Texas Medical Branch, 301 University Blvd, Galveston, TX 77555-0609, USA
Top