Volume 13, Number 1—January 2007
Research
Primary Pneumocystis Infection in Infants Hospitalized with Acute Respiratory Tract Infection
Cite This Article
Citation for Media
Abstract
Acquisition of Pneumocystis jirovecii infection early in life has been confirmed by serologic studies. However, no evidence of clinical illness correlated with the primary infection has been found in immunocompetent children. We analyzed 458 nasopharyngeal aspirates from 422 patients hospitalized with 431 episodes of acute respiratory tract infection (RTI) by using a real-time PCR assay. In 68 episodes in 67 infants, P. jirovecii was identified. The odds ratio (95% confidence interval) of a positive signal compared with the first quartile of age (7–49 days) was 47.4 (11.0–203), 8.7 (1.9–39.7), and 0.6 (0.1–6.7) for infants in the second (50–112 days), third (113–265 days), and fourth (268–4,430 days) age quartiles, respectively. Infants with an episode of upper RTI (URTI) were 2.0 (1.05–3.82) times more likely to harbor P. jirovecii than infants with a lower RTI. P. jirovecii may manifest itself as a self-limiting URTI in infants, predominantly those 1.5–4 months of age.
The opportunistic fungus Pneumocystis jirovecii (formerly Pneumocystis carinii f.sp. hominis [1]) may cause severe pneumonia (PCP) in patients with AIDS and other immunodeficiencies. The epidemiology of P. jirovecii infection is still not well understood, however. Serologic studies have shown that children are exposed to P. jirovecii early in life (2–5).
To our knowledge, no previous evidence exists of a correlation between clinical illness and primary infection in the competent host (6). Recently, P. jirovecii has been found in respiratory secretions from infants with respiratory tract infection (RTI) as well as in autopsy lung tissue from infants who died of sudden infant death syndrome (7–10).
The role of a human reservoir for the pathogen, consisting of HIV-positive or HIV-negative adults, has recently been debated (11). Also, immunocompetent children may contribute to the circulation of the organism. In addition, detecting abundant infection in infants could reflect widespread exposure from an environmental reservoir.
We conducted a blinded, retrospective study to determine the prevalence of P. jirovecii harbored in the respiratory tracts of Danish children with acute RTI, and whether clinical and laboratory characteristics separate those with and without P. jirovecii infection. The detection method employed was a single-round, closed-tube, real-time PCR assay. The study was approved by the Ethical Committee of Copenhagen (KF 01–028/03).
Patient Population and Samples
All available routine nasopharyngeal aspirates (NPAs) obtained for respiratory syncytial virus (RSV) analysis during 1999–2002 from children hospitalized at the Departments of Pediatrics, Hvidovre University Hospital and Amager Hospital, Copenhagen, Denmark, were included in the study. Thus, included samples and subjects were NPAs from children in whom the treating physician suspected or wished to rule out an RSV infection. Therefore, most children were <24 months of age. Samples collected within 3 weeks from the same person were regarded as being from the same episode of respiratory disease.
Clinical Data Collection
Clinical data were obtained by reviewing medical records of the patients using uniform data abstraction forms. The reviewer, a pediatrician, was blinded to PCR data. A diagnosis of lower RTI (LRTI), upper RTI (URTI), or “other” was made on the basis of recorded clinical findings (12). In brief, a diagnosis of URTI was made if the infant had one or more of the following clinical signs without evidence of LRTI: cough, nasal discharge, a red bulging tympanic membrane, and pharyngotonsillar erythema or exudate. A diagnosis of LRTI was made if the infant also had abnormal sounds on lung auscultation and chest indrawing or tachypnea. Infants with an RTI who were hospitalized primarily for other reasons received the diagnosis “other.”
PCR Analysis
All samples were extracted and assayed in the Clinical Microbiology Laboratory at Hvidovre University Hospital. Universal PCR laboratory procedures were used, such as physical separation of the steps involved in PCR and unidirectional workflow; specimens were processed carefully with observance of universal PCR laboratory precautions. In addition, a single-round, nonnested, closed-tube PCR assay, with no manipulations of amplicons required, inherently reduces the risks of carryover contamination. To further reduce this risk, uracil-
DNA Extraction
DNA was extracted from patient and control specimens with the automated MagnaPure (Roche Diagnostics GmbH, Mannheim, Germany) system, using the MagNA Pure LC DNA Kit III (bacteria, fungi) (Roche), according to the manufacturer’s recommendations. A sample volume of 100 μL was used for extraction, and a preincubation step was carried out by adding lysis buffer and protein K mixture to the sample, which was then incubated for 15 min at 65°C, followed by 10 min at 95°C. Extracted material was stored at −80°C.
PCR Controls
P. jirovecii–positive and –negative respiratory samples, as determined by results of previous microscopy and PCR, were included with each DNA extraction and in each PCR run as external controls. An internal control amplifiable by the P. jirovecii primers was included to detect PCR inhibitors in the patient specimens (14).
The following standards were set. For Pneumocystis, 10-fold serial dilutions (10−2–105 copies/μL) of a plasmid containing a P. jirovecii major surface glycoprotein (MSG) gene insert were prepared (14). Standard curves for quantification of positive patient samples were generated by assaying the serial dilution in triplicate. For betaglobin, 10-fold serial dilutions (1.5 × 10−4–1.5 × 101 ng/μL]) of human genomic DNA provided with the Control Kit DNA (Roche) were used to generate standard curves, according to the manufacturer’s instructions.
DNA amplification and detection were carried out as follows. For Pneumocystis, we used a quantitative touchdown PCR method that targeted the multicopy MSG gene of P. jirovecii (14). In brief, primers JKK14/15 and JKK17 amplify a 250-bp segment of the multicopy MSG gene family. The MSG primers also amplify a 295-bp fragment of the artificially constructed internal control. Detection was carried out by using 2 separate sets of fluorescence resonance energy transfer (FRET) probes, which detected the MSG (PCMSGFRET1U and PCMSGFRET1D) and internal control target (PCMIM1U and PCMIM1D), respectively. The probes were labeled with Red640 and Red705, respectively, for simultaneous amplification and detection to take place in the same reaction tube. PCR conditions were as previously described (14). First, all samples were assayed with the internal control included. P. jirovecii–positive samples were then assayed for quantification without an internal control, including 2 standards (103 copies/μL) in the experiment, and the generated external standard curve was imported for quantification.
For betaglobin, a commercial kit, Control Kit DNA (Roche), was used to estimate the amount of human DNA present in the samples. PCR conditions were as recommended by the company. Two standards (1.5 × 10−1 ng/μL) were included in each experiment, and the generated external standard curve was imported for quantification. All P. jirovecii–positive samples and a randomly selected subgroup of P. jirovecii–negative samples (all negative samples from patients born on the first through third days of the month) were assayed; 5 μL of patient specimen or the standard dilution was added per tube.
Interpretation
If a PCR-positive sample was negative by the second analysis, the sample was reextracted and reassayed in 2 tubes. If at least 2 of 4 tubes were positive, the sample was recorded as positive for P. jirovecii.
A negative MSG result had to have a positive result for the internal control to be considered valid, to ensure absence of inhibitors in the specimen. If PCR inhibitors were detected, the sample was to be diluted 1:5.
Data Analysis
All acquired fluorescence data were analyzed with LightCycler software (Roche). Clinical data were recorded with EpiData 2.1a (EpiData Association, Odense, Denmark). Statistics were calculated by using the SAS System, version 9.1 for Windows (SAS Institute Inc., Cary, NC, USA).
Wilcoxon 2-sample test or Kruskal-Wallis test was used to compare quantitative data when appropriate. Fisher exact test was used to compare groups. A 2-sided p value of <0.05 was considered significant. Values presented are medians with ranges or interquartile ranges (IQR). Logistic regression was used for univariate and multivariate analyses.
Patients and Episodes
Four hundred sixty-one NPAs from 423 patients were available for analysis. One HIV-infected child with PCP was excluded from analysis. The remaining infants were presumed to be uninfected with HIV of the basis of review of their medical charts. Two hundred ninety-six (70%) patients (with 303 episodes [70%]) were hospitalized at Hvidovre University Hospital and the rest at Amager Hospital. Sixty-four percent of the episodes received a diagnosis of LRTI, 28% a diagnosis of URTI, and 8% “other.” The median age was 112 days (IQR 49–265), and 52.7% of the NPAs were from male patients.
PCR Results
No samples exhibited inhibition. All controls were appropriate. Sixty-seven (16%) of the 422 patients had positive test results for P. jirovecii in 68 (16%) of 431 episodes. More than 1 NPA was collected in 21 episodes, of which 4 (19%) were P. jirovecii positive, and PCR results were concordant in 96% (46/48 samples) of the NPAs. NPAs from 8 pairs of siblings were collected, and all pairs were concordant (1/8 pairs positive).
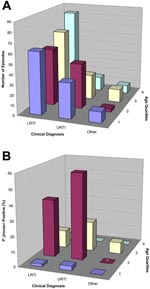
Basic demographic data for the P. jirovecii–positive and –negative groups are presented in Table 1, and age distribution in quartiles is presented in Table 2. Significant differences were found in age, days admitted to hospital, and occurrence of reported fever. However, no significant difference was found in temperature at admission. No difference in positivity rate was seen between the 2 hospitals. Univariate and multivariate analyses are presented in Table 3. By univariate analysis, URTI versus LRTI, age quartiles 2 and 3 versus 1, and reported fever were associated with the presence of P. jirovecii but days admitted to the hospital was not. Age quartiles 2 and 3 versus quartile 1 and URTI versus LRTI were independently associated with P. jirovecii positivity by multivariate analysis. The distribution of number of episodes by clinical diagnosis and age is illustrated by Appendix Figure A and the frequency of P. jirovecii–positive episodes by Appendix Figure B.
Quantitative Analysis of PCR-positive Results
If >1 NPAs were collected during the same episode, average numbers of copies were calculated for that episode. The P. jirovecii–positive episodes had a median of 9 copies/tube (IQR 2.8–25).
Of the 387 P. jirovecii–negative NPAs, 49 (12.7%) were randomly selected for betaglobin analysis. The P. jirovecii–positive and –negative samples had a median of 129,400 (IQR 49,540–298,800) versus 95,410 (IQR 27,610–228,800) pg/tube, with no significant difference (p = 0.09).
Due to the natural variation of the specimens, P. jirovecii copy numbers were corrected for amount of human DNA in the sample (copies MSG per ng betaglobin). The PCR-positive episodes had a median of 0.069 (IQR 0.021–0.315) copies/ng betaglobin per tube.
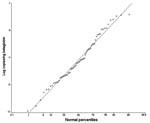
The quantitative data were normally distributed when logarithm transformed (Figure). Quantitative data for age and clinical diagnosis subgroups are presented in Table 4. No significant differences were found among groups.
In this study, P. jirovecii was detected in NPAs from 16% of infants hospitalized with acute RTI. A marked difference occurred in the age distribution, as the prevalence was 48% in infants ages 50 to 112 days (second quartile), 13% in infants ages 113 to 265 days (third quartile), and negligible in the youngest and oldest infants (Table 2, Appendix Figure). Similarly, ORs of 47 and 8.7 were found for the second and third quartiles, respectively, when compared with that of the youngest group for being P. jirovecii positive by multivariate analysis (Table 3). These data indicate that infants were exposed very early to P. jirovecii, and this raises the question of whether this diagnosis should be considered in infants ages 1.5–4 months who exhibit symptoms of an acute RTI.
The relative absence of P. jirovecii among the youngest infants (ages <50 days) could indicate either differences in exposure or in immunity, or reflect the incubation time of the infection. One could hypothesize that the increased rate of P. jirovecii positivity was coincidental with the infants’ introduction to a daycare facility/institution. However, the infants were cared for at home and not at an institution in 64 (96%) of the 67 P. jirovecii–positive episodes.
Another possible explanation is differences in immunity, which could be mediated by maternal antibodies in the youngest infants. Animal studies have shown that maternal antibodies are protective in infants (15–17). Likewise, P. jirovecii was seldom found in the oldest infants (>265 days of age), which may have been due to acquired immunity. Previous studies suggest that the clearance of organisms is complete; no detectable organisms were found by microscopy or PCR in postmortem lung specimens from immunocompetent adult patients (18). However, primary infection could possibly be acquired later in life and produce a milder illness (one that does not require hospitalization) in older children, and therefore these cases are not included in the current study.
Also, the absence of P. jirovecii among the youngest infants (ages <50 days) may have been a result of the incubation period of the infection, assuming that organism burden during the incubation period was below level of detection of the assay. Animal studies have indicated that the peak organism load in healthy mice occurs 5–6 weeks after exposure (19,20). Thus, the infants could have been exposed shortly after birth in order for symptoms to develop in infants at the ages found here. In fact, animal studies have found early exposure of newborn infants by a maternal source, and asymptomatic carriage by pregnant women has been reported recently (21–23). In a reported case of probable mother-to-infant transmission, the mother became symptomatic at 3 days postpartum and the infant at 29 days of age (24). The shorter incubation period in this case may reflect a higher level of infectious inoculum in this infant.
The age distribution was in concordance with the trend reported in a recent study on autopsied lung specimens from 112 infants (25). Similarly, serologic studies have indicated that most children seroconvert early in life (2–5,8,26). Among children with perinatally acquired HIV, the incidence of PCP was highest from 3 to 6 months of age (27,28). That is, these infants were slightly older when PCP was diagnosed. Assuming that they were exposed to P. jirovecii at the same time as healthy infants, the difference could be because a longer incubation time is needed for clinical PCP to develop in susceptible immunocompromised persons. Previous studies have reported an overall P. jirovecii prevalence of 25% (8), and 32% (9) in infants with acute RTI. When episodes were considered, however, the prevalence in the latter study was 17%, which was in concordance with the findings in our current study. The former study comprised 178 infants but did not include clinical data, and the latter study comprised a smaller population (74 infants with 178 episodes). Geographic variation or methodologic differences may account for the slight difference in reported prevalence. Our study used a single-round, closed-tube, PCR format for detection, which has a high sensitivity and a greatly reduced risk for carryover contamination (14). The difference in amount of human DNA detected in P. jirovecii–positive and –negative samples did not reach the 5% level of significance. If, in fact, a difference exists, this could be because sample quality varied, which means that we may have underestimated the true prevalence of P. jirovecii carriage. The difference could also have occurred because the presence of P. jirovecii increases the amount of, for example, inflammatory cells in the nasopharyngeal secretions (29), thereby increasing the amount of human DNA sampled. The current study confirms the previous reports that P. jirovecii can be detected in respiratory tract specimens from otherwise healthy infants with an acute RTI.
Pneumocystis is likely transmitted through the respiratory route (30). The reservoir for P. jirovecii is unknown but could include other persons or environmental sources, whereas animal reservoirs are unlikely because of the host specificity (6,31). Animal studies have shown that colonized mice may transmit the organism to immunosuppressed mice (32). Therefore, healthy children with a primary P. jirovecii infection may play a role in the circulation of the organism as previously suggested (25), although recent genotyping studies have yielded conflicting results (10,33).
P. jirovecii–positive episodes could represent either colonization or clinical overt disease. We have previously shown the assay used here provides reproducible quantitative results, and that a similar real-time quantitative PCR assay correlates well with the number of whole organisms in the sample (14,34). The fact that the quantitative data were normally distributed after logarithmic transformation (Figure), and that no differences in copy numbers were detected among groups (Table 4), may indicate that the P. jirovecii–positive episodes represent 1 biologic phenomenon.
To our knowledge, no previous evidence has shown a connection between clinical illness or specific symptoms and primary infection in children. It has been presumed to be an asymptomatic or mild, nonspecific disease (6,22). In the study by Vargas et al., no differences in clinical diagnosis were observed (8). In the current study, we found that infants with an episode of URTI were 2.0× more likely to be carrying P. jirovecii than infants with LRTI, when findings were adjusted for age (Appendix Figure, Table 3). This finding is somewhat surprising because the organism causes LRTI in immunocompromised subjects. It is unlikely that the finding is due to differences in sample quality, because the amount of betaglobin detected in samples from patients with URTI and LRTI was similar (data not shown), and no difference in adjusted Pneumocystis DNA was detected (Table 4). Parents reported that the child had a history of fever less often in P. jirovecii–positive episodes by univariate analysis, but no differences were found in the presence of fever as assessed at admission (Tables 1 and 3). Also, P. jirovecii–positive infants tended to be hospitalized for a marginally shorter duration (Tables 1 and 3).
The limitations of this study are primarily the lack of a healthy control group without respiratory symptoms, and lack of serologic data from the patients. Also, a comprehensive analysis of the specimens was not done for known respiratory pathogens other than RSV and P. jirovecii. Further investigation is therefore needed to confirm these findings before recommendations can be made for routine diagnostic testing for Pneumocystis in defined populations of infants, because it remains possible that Pneumocystis carriage in this population could represent a bystander phenomenon. Similarly, one should be cautious in inferring these results to infants without RTI or to those with an RTI that does not require hospitalization.
In this study, we found an overall prevalence of P. jirovecii in the respiratory tracts of 16% of infants hospitalized with an episode of acute RTI. Infants ages 50–112 days harbored P. jirovecii in 48% of the episodes. Our data suggest that primary P. jirovecii infection acquired early in life may present itself as a self-limiting URTI.
Dr Larsen is currently a fellow in the Department of Bacteriology, Mycology and Parasitology, State Serum Institute, Copenhagen, Denmark. His primary research interest is the epidemiology and diagnosis of Pneumocystis infections.
Acknowledgments
We thank Lena Hansen for technical assistance.
Hans H. Larsen was supported by Copenhagen HIV Programme; Danish Hospital Foundation for Medical Research, Region of Copenhagen; the Faroe Islands, and Greenland; the Danish Lung Association; and the Beckett Foundation.
References
- Redhead SA, Cushion MT, Frenkel JK, Stringer JR. Pneumocystis and Trypanosoma cruzi: nomenclature and typifications.J Eukaryot Microbiol. 2006;53:2–11. DOIPubMedGoogle Scholar
- Lundgren B, Lebech M, Lind K, Nielsen JO, Lundgren JD. Antibody response to a major human Pneumocystis carinii surface antigen in patients without evidence of immunosuppression and in patients with suspected atypical pneumonia.Eur J Clin Microbiol Infect Dis. 1993;12:105–9. DOIPubMedGoogle Scholar
- Peglow SL, Smulian AG, Linke MJ, Pogue CL, Nurre S, Crisler J, Serologic responses to Pneumocystis carinii antigens in health and disease.J Infect Dis. 1990;161:296–306.PubMedGoogle Scholar
- Pifer LL, Hughes WT, Stagno S, Woods D. Pneumocystis carinii infection: evidence for high prevalence in normal and immunosuppressed children.Pediatrics. 1978;61:35–41.PubMedGoogle Scholar
- Wakefield AE, Stewart TJ, Moxon ER, Marsh K, Hopkin JM. Infection with Pneumocystis carinii is prevalent in healthy Gambian children.Trans R Soc Trop Med Hyg. 1990;84:800–2. DOIPubMedGoogle Scholar
- Kovacs JA, Gill VJ, Meshnick S, Masur H. New insights into transmission, diagnosis, and drug treatment of Pneumocystis carinii pneumonia.JAMA. 2001;286:2450–60. DOIPubMedGoogle Scholar
- Vargas SL, Ponce CA, Hughes WT, Wakefield AE, Weitz JC, Donoso S, Association of primary Pneumocystis carinii infection and sudden infant death syndrome.Clin Infect Dis. 1999;29:1489–93. DOIPubMedGoogle Scholar
- Vargas SL, Hughes WT, Santolaya ME, Ulloa AV, Ponce CA, Cabrera CE, Search for primary infection by Pneumocystis carinii in a cohort of normal, healthy infants.Clin Infect Dis. 2001;32:855–61. DOIPubMedGoogle Scholar
- Nevez G, Totet A, Pautard JC, Raccurt C. Pneumocystis carinii detection using nested-PCR in nasopharyngeal aspirates of immunocompetent infants with bronchiolitis.J Eukaryot Microbiol. 2001;Suppl:122S–3S. DOIPubMedGoogle Scholar
- Beard CB, Fox MR, Lawrence GG, Guarner J, Hanzlick RL, Huang L, Genetic differences in Pneumocystis isolates recovered from immunocompetent infants and from adults with AIDS: epidemiological implications.J Infect Dis. 2005;192:1815–8. DOIPubMedGoogle Scholar
- Morris A, Lundgren JD, Masur H, Walzer PD, Hanson DL, Frederick T, Current epidemiology of Pneumocystis pneumonia.Emerg Infect Dis. 2004;10:1713–20.PubMedGoogle Scholar
- von Linstow ML, Larsen HH, Eugen-Olsen J, Koch A, Nordmann WT, Meyer AM, Human metapneumovirus and respiratory syncytial virus in hospitalized Danish children with acute respiratory tract infection.Scand J Infect Dis. 2004;36:578–84. DOIPubMedGoogle Scholar
- Sobek H, Schmidt M, Frey B, Kaluza K. Heat-labile uracil-DNA glycosylase: purification and characterization.FEBS Lett. 1996;388:1–4. DOIPubMedGoogle Scholar
- Larsen HH, Masur H, Kovacs JA, Gill VJ, Silcott VA, Kogulan P, Development and evaluation of a quantitative, touch-down, real-time PCR assay for diagnosing Pneumocystis carinii pneumonia.J Clin Microbiol. 2002;40:490–4. DOIPubMedGoogle Scholar
- Bartlett MS, Angus WC, Shaw MM, Durant PJ, Lee CH, Pascale JM, Antibody to Pneumocystis carinii protects rats and mice from developing pneumonia.Clin Diagn Lab Immunol. 1998;5:74–7.PubMedGoogle Scholar
- Empey KM, Hollifield M, Schuer K, Gigliotti F, Garvy BA. Passive immunization of neonatal mice against Pneumocystis carinii f. sp. muris enhances control of infection without stimulating inflammation.Infect Immun. 2004;72:6211–20. DOIPubMedGoogle Scholar
- Garvy BA, Harmsen AG. Susceptibility to Pneumocystis carinii infection: host responses of neonatal mice from immune or naive mothers and of immune or naive adults.Infect Immun. 1996;64:3987–92.PubMedGoogle Scholar
- Peters SE, Wakefield AE, Sinclair K, Millard PR, Hopkin JM. A search for Pneumocystis carinii in post-mortem lungs by DNA amplification.J Pathol. 1992;166:195–8. DOIPubMedGoogle Scholar
- Vestereng VH, Bishop LR, Hernandez B, Kutty G, Larsen HH, Kovacs JA. Quantitative real-time polymerase chain-reaction assay allows characterization of pneumocystis infection in immunocompetent mice.J Infect Dis. 2004;189:1540–4. DOIPubMedGoogle Scholar
- An CL, Gigliotti F, Harmsen AG. Exposure of immunocompetent adult mice to Pneumocystis carinii f. sp. muris by cohousing: growth of P. carinii f. sp. muris and host immune response.Infect Immun. 2003;71:2065–70. DOIPubMedGoogle Scholar
- Icenhour CR, Rebholz SL, Collins MS, Cushion MT. Early acquisition of Pneumocystis carinii in neonatal rats as evidenced by PCR and oral swabs.Eukaryot Cell. 2002;1:414–9. DOIPubMedGoogle Scholar
- Peterson JC, Cushion MT. Pneumocystis: not just pneumonia.Curr Opin Microbiol. 2005;8:393–8. DOIPubMedGoogle Scholar
- Vargas SL, Ponce CA, Sanchez CA, Ulloa AV, Bustamante R, Juarez G. Pregnancy and asymptomatic carriage of Pneumocystis jiroveci.Emerg Infect Dis. 2003;9:605–6.PubMedGoogle Scholar
- Miller RF, Ambrose HE, Novelli V, Wakefield AE. Probable mother-to-infant transmission of Pneumocystis carinii f. sp. hominis infection.J Clin Microbiol. 2002;40:1555–7. DOIPubMedGoogle Scholar
- Vargas SL, Ponce CA, Luchsinger V, Silva C, Gallo M, Lopez R, Detection of Pneumocystis carinii f. sp. hominis and viruses in presumably immunocompetent infants who died in the hospital or in the community.J Infect Dis. 2005;191:122–6. DOIPubMedGoogle Scholar
- Respaldiza N, Medrano FJ, Medrano AC, Varela JM, de La HC, Montes-Cano M, High seroprevalence of Pneumocystis infection in Spanish children.Clin Microbiol Infect. 2004;10:1029–31. DOIPubMedGoogle Scholar
- Simonds RJ, Oxtoby MJ, Caldwell MB, Gwinn ML, Rogers MF. Pneumocystis carinii pneumonia among US children with perinatally acquired HIV infection.JAMA. 1993;270:470–3. DOIPubMedGoogle Scholar
- Ruffini DD, Madhi SA. The high burden of Pneumocystis carinii pneumonia in African HIV-1-infected children hospitalized for severe pneumonia.AIDS. 2002;16:105–12. DOIPubMedGoogle Scholar
- Vestbo J, Nielsen TL, Junge J, Lundgren JD. Amount of Pneumocystis carinii and degree of acute lung inflammation in HIV-associated P. carinii pneumonia.Chest. 1993;104:109–13. DOIPubMedGoogle Scholar
- Hughes WT. Natural mode of acquisition for de novo infection with Pneumocystis carinii.J Infect Dis. 1982;145:842–8.PubMedGoogle Scholar
- Gigliotti F, Harmsen AG, Haidaris CG, Haidaris PJ. Pneumocystis carinii is not universally transmissible between mammalian species.Infect Immun. 1993;61:2886–90.PubMedGoogle Scholar
- Dumoulin A, Mazars E, Seguy N, Gargallo-Viola D, Vargas S, Cailliez JC, Transmission of Pneumocystis carinii disease from immunocompetent contacts of infected hosts to susceptible hosts.Eur J Clin Microbiol Infect Dis. 2000;19:671–8. DOIPubMedGoogle Scholar
- Totet A, Latouche S, Lacube P, Pautard JC, Jounieaux V, Raccurt C, Pneumocystis jirovecii dihydropteroate synthase genotypes in immunocompetent infants and immunosuppressed adults, Amiens, France.Emerg Infect Dis. 2004;10:667–73.PubMedGoogle Scholar
- Larsen HH, Kovacs JA, Stock F, Vestereng VH, Lundgren B, Fischer SH, Development of a rapid real-time PCR assay for quantitation of Pneumocystis carinii f. sp. carinii.J Clin Microbiol. 2002;40:2989–93. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 13, Number 1—January 2007
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Hans Henrik Larsen, Dept. Clinical Microbiology 9301, Rigshospitalet, Juliane Maries Vej 22, 2100 Copenhagen, Denmark;
Top