Volume 13, Number 11—November 2007
Research
Protection and Virus Shedding of Falcons Vaccinated against Highly Pathogenic Avian Influenza A Virus (H5N1)
Cite This Article
Citation for Media
Abstract
Because fatal infections with highly pathogenic avian influenza A (HPAI) virus subtype H5N1 have been reported in birds of prey, we sought to determine detailed information about the birds’ susceptibility and protection after vaccination. Ten falcons vaccinated with an inactivated influenza virus (H5N2) vaccine seroconverted. We then challenged 5 vaccinated and 5 nonvaccinated falcons with HPAI (H5N1). All vaccinated birds survived; all unvaccinated birds died within 5 days. For the nonvaccinated birds, histopathologic examination showed tissue degeneration and necrosis, immunohistochemical techniques showed influenza virus antigen in affected tissues, and these birds shed high levels of infectious virus from the oropharynx and cloaca. Vaccinated birds showed no influenza virus antigen in tissues and shed virus at lower titers from the oropharynx only. Vaccination could protect these valuable birds and, through reduced virus shedding, reduce risk for transmission to other avian species and humans.
Highly pathogenic avian influenza A (HPAI) virus poses a major threat to poultry but is also of great concern for other avian species and humans. In particular, HPAI (H5N1) of Asian lineage is known for its potential to be transmitted to mammals, including humans. Susceptibility to this virus and the possible role as vectors or reservoirs vary greatly between different wild bird and poultry species (1,2). Gallinaceous poultry are considered to be highly susceptible, whereas waterfowl may show variable clinical signs depending on the strain of infecting virus. Birds of prey are at increased risk for infection with HPAI virus because they regularly feed on avian carcasses and diseased avian prey (3,4). Many species are migratory or cover an extensive territory and may spread the virus within or between countries. In falconry, birds of prey are also regularly kept in captivity and come in close contact with humans. In this respect, birds of prey represent a bridging species and may pose a risk of transmitting the virus to humans or to other captive avian species, including poultry.
In the past, HPAI rarely occurred in birds of prey and only in isolated cases. In 2000, Manvell et al. (5) isolated influenza virus (H7N3) from a Peregrine falcon (Falco peregrinus) kept as a falconry bird in the United Arab Emirates. In the same year, during an HPAI (H7N7) outbreak in poultry in Italy, an avian influenza virus of H7 subtype was isolated from a Saker falcon (Falco cherrug) (6). Both birds showed depression and died, but other pathogens (e.g., Pasteurella sp.) were detected as well.
During recent influenza (H5N1) outbreaks, increasing numbers of birds of prey were reported to be infected. HPAI virus (H5N1) was isolated from Hodgson’s hawk eagles (Spizaetus nipalensis) confiscated at an airport (7) and from a Saker falcon (8) in Saudi Arabia. During the influenza (H5N1) outbreak among wild birds in Germany, 36 (10.5%) birds with positive influenza (H5N1) results were birds of prey, represented by common buzzards (Buteo buteo), peregrines (F. peregrinus), and kestrels (Falco tinnunculus), as well as European eagle owls (Bubo bubo), which were found dead (9). Diseased free-ranging birds of prey infected with influenza (H5N1) were also reported by several other countries. In March 2007, influenza virus (H5N1) was isolated from falcons in Kuwait (www.poultrymed.com).
Although it is obvious that birds of prey can be infected with HPAI viruses, the pathogenic potential in these species remains unclear. Free-ranging birds frequently suffer from other concurrent diseases or starvation, and captive birds undergo stressful periods due to rearing conditions or training. These situations may immunocompromise the birds, leading to increased vulnerability. However, their potential to shed virus after infection, which is important for virus transmission, potentially also to humans, remains unclear. Clinical signs, pathologic and histopathologic alterations, and tissue tropism of the virus after a controlled infection have not been investigated. This knowledge is needed for a better understanding of HPAI in nondomestic birds, especially for subtype H5N1, which poses a higher risk to humans than do other avian influenza viruses (10–12).
Collections of birds of prey are of high commercial and species conservation value; therefore, protection from HPAI is important. Vaccination might reduce the risk for virus transmission by reducing virus shedding, as has been shown in chickens (13,14). Ultimately, an interruption of virus transmission between and within avian collections would be invaluable for controlling disease, especially in populations of rare species as exemplified by many bird of prey species (15).
Vaccination with commercially available inactivated vaccines based on avian influenza virus subtype H5 can confer clinical protection and reduce virus shedding after infection (16). Implementation of DIVA (Differentiating Infected from Vaccinated Animals) strategies have been attempted (17). Response to vaccination of zoo birds with an AI H5N2 (18) or H5N9 (19) subtype inactivated vaccine varied considerably among species with respect to peak titers and persistence of specific antibodies. Some species mounted antibodies after the first round of vaccination; others had detectable titers only after a second dose or never produced detectable antibody levels (pelicans [Pelicanus spp.] and owls [B. bubo, Tyto alba]) (20). The authors demonstrated peak hemagglutination inhibition (HI) titers of 2,048 within 2–4 weeks after booster vaccination in bar-headed geese (Anser indicus); most other species yielded titers of only 64 to 512 during the same time. Several species, such as the Egyptian goose (Alopochen aegyptiacus) and peafowl (Acryllium vulturinum), still had antibody titers of 32 to 128 by 6 months after vaccination, while spur-winged geese (Plectropterus gambensis) failed to show titers after that time. Such a variation between species was also observed after vaccination of different waterfowl and wader species (21).
No detailed information is available about antibody responses and protection after vaccination against HPAI in falcons. Therefore, we analyzed the susceptibility of falcons to an influenza (H5N1) field virus under controlled conditions and evaluated the efficacy of vaccination of falcons with an inactivated influenza (H5N2) vaccine and its effect on epidemiologically relevant parameters. The trial was approved under government registration numbers G 0072/06 and LVL M-V/TSD/7221.3-1.1-45/05 (with expansion LVL M-V/TSD/7221.3-1.1-37/06).
Animals
Fifteen juvenile female Gyr-Saker (F. rusticolus × F. cherrug) hybrid falcons were obtained from 1 breeder. The birds received an intensive health evaluation, which included a general examination, radiographs, laparoscopy, blood cell count, blood chemistry analysis, and parasitologic examination; all results were within normal limits. The falcons were perched according to standard falconry techniques during the vaccination trial (22). For challenge infection, the animals were kept individually in stainless steel cages located in negatively pressurized isolation rooms within Biosafety Level 3 facilities. Seven 1-day-old chicks obtained from a disease-free stock, were provided to each bird each day as feed. Unconsumed chicks were removed to measure the daily feed intake of each bird.
Vaccination
Ten falcons were vaccinated (nos. 1–5 intramuscularly and nos. 6–10 subcutaneously) with 0.5 mL (hemagglutinating titer >16) of influenza (H5N2) inactivated vaccine (Intervet, Unterschleissheim, Germany) based on strain A/duck/Potsdam/1402/86; they were revaccinated with the same dose and by the same route 4 weeks later. As a negative control, 5 nonvaccinated falcons were kept with the vaccinated birds.
Before the first vaccination and in weekly intervals until 8 weeks after initial vaccination, individual blood samples were collected from the metatarsalis plantaris superficialis medialis vein directly into a serum tube (Sarstedt, Nümbrecht, Germany) by using a 0.7-mm × 30-mm needle (Sterican, Luer-Lock, Braun Melsungen, Germany). The serum was separated and tested for H5-specific antibodies by the HI test, with low pathogenic avian influenza subtype H5N2 (A/duck/Potsdam/619/85) as antigen according to standardized methods (23). A cloacal swab was also examined for influenza A virus RNA by using real-time reverse transcription–PCR (RT-PCR) targeting an M gene fragment to exclude a concurrent infection (24).
Challenge Infection
Five months after the initial vaccination, 5 falcons randomly selected from the 10 vaccinated birds (nos. 1, 2, 5, 8, 9) and 5 nonvaccinated control birds (nos. 11–15) were challenged with 106.0 50% egg infectious dose (EID50) of influenza strain A/Cygnus cygnus/Germany/R65/2006, a highly pathogenic H5N1 strain that was isolated from a dead whooper swan (Cygnus cygnus) during an outbreak of HPAI virus (H5N1) among wild birds in Germany (25). Each bird received 1 mL cell culture medium by the oculo-oronasal route. The falcons were observed daily for 11 days after challenge. At the end of the trial, surviving birds were humanely killed. A serum sample was obtained by using the above-described method just before challenge and, for surviving birds, on the last day of the trial. The serum was used for the detection of antibodies against H5 by the HI test (see above) by using 2 different antigens (challenge and vaccine strain). Before challenge and at days 1, 2, 4, 7, and 11 after challenge, an oropharyngeal and a cloacal swab were taken for a semiquantitative detection of avian influenza virus–specific RNA by using a real-time RT-PCR targeting an M gene fragment as recommended in the Diagnostic Manual for Avian Influenza issued by the European Commission (26) and described by Spackman et al. (24). The method has been improved by using an internal control in parallel in a duplex reaction (27). In addition, virus isolation in embryonated chicken eggs was attempted as described by Werner et al. (28). Isolated viruses were characterized as HPAI virus (H5N1) by subtype-specific real-time RT-PCRs (26) and by a pathotype-specific real-time RT-PCR (29).
Gross, Histopathologic, and Immunohistochemical Examinations
Necropsies were performed immediately after death. Samples of nasal cavity, trachea, lung, heart, cerebellum, cerebrum, spinal cord, proventriculus, small and large intestine, liver, pancreas, spleen, skin, and kidney were collected and either snap frozen or formalin fixed (48 h) and processed for paraffin embedding according to standardized procedures. For histopathologic examination, paraffin wax sections (3 µm) were dewaxed and stained with hematoxylin and eosin. Immunohistochemical examination for influenza virus A nucleoprotein (NP) was performed according to Klopfleisch et al. (30). Briefly, dewaxed sections were incubated with a rabbit anti-NP serum (1:500). As secondary antibody, biotinylated goat anti-rabbit IgG1 (Vector, Burlingame, CA, USA) was applied. By means of the avidin-biotin-peroxidase complex method, a bright red signal was produced. Positive and negative control tissues of chickens that had been infected experimentally with HPAI virus (H5N1) were included. Tissues from the central nervous system (CNS), small intestine, pancreas, trachea, and lung were used for real-time RT-PCR and for virus isolation.
Immune Response
During the entire trial, control birds remained negative for avian influenza virus H5-specific antibodies. In addition, influenza A virus RNA was not detected in any of the cloacal swabs. No adverse clinical effects were detected as a result of application of the 2 vaccine doses.
Nine of the 10 vaccinated birds mounted homologous H5-specific antibodies 3 weeks after the first vaccination; titers increased significantly after the booster vaccination (Table 1). The remaining bird (no. 5) showed a detectable titer of 8 only 6 weeks after initial vaccination (2 weeks after booster vaccination); HI titer for this bird remained at 8. Differences in titer development according to route of vaccination were not detected (Table 1). Clinical signs (i.e., decreased food intake or worsening general condition) were not observed in any of the vaccinated birds. HI titers against the heterologous challenge strain A/Cygnus cygnus/Germany/R65/2006 at the time of challenge are shown in Figure 1. The nonvaccinated birds remained seronegative.
Gross, Histopathologic, and Immunohistochemical Response to Challenge
All nonvaccinated birds died after challenge with HPAI virus (H5N1). The first falcon died on day 3 postchallenge, 3 died on day 4, and the rest died at day 5. Of these, 4 had reduced food intake starting from the day of challenge, and 3 had a slightly bloody tracheal exudate detectable the day after exposure. One bird died with no clinical signs.
All vaccinated birds survived. For 2, food intake was slightly reduced 1 day after challenge. No other vaccinated bird exhibited clinical signs. By 11 days after challenge, the titers of the vaccinated birds increased to 2,048 against the antigen used for vaccination and 1,024 against the challenge strain (Figure 1).
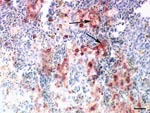
Necropsy showed multifocal acute hemorrhagic necrosis in the pancreas of 3 birds that died spontaneously and moderate to severe splenic hyperplasia in 3 birds. Histopathologic examination of the cerebellum, cerebrum, spinal cord, pancreas, spleen, and kidney of the nonvaccinated birds showed multifocal acute cellular degeneration and necrosis associated with minimal to mild infiltration of few heterophils and detection of HPAI virus antigen (Figure 2). Furthermore, antigen was present in the nasal cavity, trachea, bronchial epithelium, and gastrointestinal tract but not in the liver and skin. None of the euthanized vaccinated birds exhibited any gross or histologic lesions or presence of antigen in any of the tissues.
Virus Excretion after Challenge
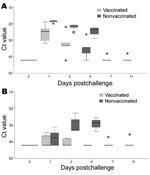
In the nonvaccinated falcons, after challenge infection viral RNA was detectable in all oropharyngeal swabs. Virus was also isolated from the pooled oropharyngeal swabs of these birds taken on the same days (Figure 3; Table 2). At day 1 postchallenge, viral RNA was detected in the cloacal swabs of 3 birds, although virus isolation failed. From day 2 postchallenge, all falcons demonstrated the presence of viral RNA and infectious virus in cloacal swabs (Figure 3; Table 2).
In the vaccinated falcons, 1 day after challenge viral RNA was detectable in all oropharyngeal swabs. At day 2 postchallenge, 1 falcon became negative for viral RNA, and at days 7 and 11, only 1 bird remained positive for viral RNA (Figure 3; Table 2). Virus isolation from a pool of all oropharyngeal swabs of all 5 vaccinated birds taken from day 1 postchallenge demonstrated a virus titer of 4.4 log10 EID50/mL at day 1 postchallenge and 1.2 log10 EID50/mL at day 2 postchallenge. From day 4 on, virus could no longer be isolated from the pooled oropharyngeal swabs. Viral RNA was only occasionally detected in cloacal swabs and completely absent in 1 bird (Figure 3, Table 2). Virus could not be isolated from any of the pools of the cloacal swabs.
Virus in Tissues
In the nonvaccinated falcons, high loads of viral RNA were detected in the CNS, duodenum, pancreas, trachea, and lung of all control birds that died after challenge (Table 3). Virus isolation from these samples was not attempted.
In the vaccinated falcons, low to moderate loads of viral RNA were demonstrated in the brain and trachea of 3 birds that were euthanized on day 11 postchallenge, in the lung of 2 birds, in the duodenum of 1 bird, and in the pancreas of 1 bird. However, virus was isolated from the trachea of only 2 birds and from the lung of 1 (Table 3). The viral RNA load was as much as 6 log10 lower than that of nonvaccinated animals.
Our study is the first, to our knowledge, to demonstrate that falcons are highly susceptible to HPAI virus (H5N1) as exemplified by strain A/Cygnus cygnus/Germany/R65/2006; all nonvaccinated birds died within 5 days after challenge. Clinical signs were mild and indicated only by a reduced food intake, which is not considered very obvious because falcons typically do not eat every day. These signs will not be seen in free-ranging birds and may be overlooked in captive animals. However, under natural conditions, more pronounced clinical signs may develop because stress situations and concurrent diseases are more likely than in captivity. Considering virus replication in the CNS, as demonstrated by immunohistochemical examination, CNS disturbances such as ataxia and disorientation might have ensued, although this is difficult to verify when birds are not allowed to fly.
The slightly bloody exudate from the trachea, noted for 3 birds at day 1 postchallenge, may pass unnoticed under field conditions. On the basis of the inconspicuous clinical signs, precisely defining the length of the incubation period is difficult. Gross lesions noted at necropsy were only mild and restricted to the pancreas and, thus, may be overlooked during routine necropsy when influenza is not suspected. The striking alterations of the pancreas are important as they were found macroscopically in 3 of the 5 birds and histopathologically in all 5 birds that died. Such lesions have also been described in mute (C. olor) and whooper swans (C. cygnus) (31), in passerines and budgerigars (32), and in emus and geese (33). The systemic virus distribution parallels that noted in water fowl during the 2006 outbreak on the Baltic Sea coast (31). Nevertheless, carnivorous birds, including buzzards, affected during an outbreak in Germany in 2006 displayed mainly a severe infection of the CNS without systemic virus distribution (unpub. data). The lack of antigen detection in the vaccinated falcons at day 11 postchallenge parallels the minimal virus shedding of the vaccinated falcons. Nevertheless, infection of cells at the site of inoculation can only be excluded by immunohistochemical examination of vaccinated animals during the first days after challenge.
All nonvaccinated falcons shed virus from the oropharynx and cloaca until death. Oropharyngeal shedding peaked at day 1 postchallenge, which might be related to reisolation of inoculum, and decreased toward day 4 postchallenge. The peak of cloacal excretion was at day 2 postchallenge, as reported for chickens (14). These findings demonstrate that after infection with influenza A (H5N1) of Asian origin, oropharyngeal swabs may be superior to cloacal swabs for diagnosing infection under field conditions. Duration of virus excretion before death was very short. Therefore, falcons may not play a major role in spreading the pathogen within or between countries, although this possibility cannot be excluded. Moreover, infected birds, like these falcons, may not be able to migrate long distances. However, because they shed a considerable amount of virus for a short time concomitant with virtual absence of overt clinical signs, captive infected falcons may pose a substantial risk for humans and other birds of high commercial and species conservation value. Therefore, measures to reduce this risk are of great importance, especially because depopulation of such birds is not a well-accepted option.
Vaccination of poultry, at least in experimental settings, can reduce virus shedding significantly after challenge, depending on the amount of antigen in the vaccine and the antigenic relationship between vaccine and virulent field virus (13,14,34,35). This study shows that vaccination is also an option in falcons. It is safe; no adverse clinical reactions were observed. High titers of specific HI antibodies were induced in most vaccinated animals and persisted for at least 5 months, which indicates that biannual revaccination may suffice. However, as in chickens, sterile immunity could not be induced as shown by continuous detection of virus excretion, particularly from the oropharynx, in vaccinated falcons after challenge infection. However, virus excretion was drastically reduced in vaccinated birds compared with nonvaccinated birds and could be detected only by sensitive real-time RT-PCR. With respect to the marked differences of virus excretion between vaccinated and nonvaccinated falcons, we note that a difference of approximately 3.3 cycle-of-threshold values corresponds to 1 log10 of viral nucleic acid copies (36). Figure 3A shows that in oropharyngeal swabs from nonvaccinated falcons, up to 3–4 log10 more viral RNA copies are present than in swabs from vaccinated falcons. The failure to isolate challenge virus from excretions of vaccinated falcons raises the question of the epidemiologic importance of the presence of viral RNA in oropharyngeal swabs (13,14). Therefore, vaccination is considered to be an important tool to prevent further major outbreaks (34). Additionally, the bird-to-human infection route of HPAI seems to require a high amount of excreted virus as well as close contact (37), which seems much more difficult to achieve with vaccinated birds. Although residual infectious virus persisted in organs of a few vaccinated birds until day 11 postchallenge, whether viral loads are sufficient for efficient transmission remains unclear. Because no viral RNA could be detected in the oropharyngeal swabs of 2 of these birds, this, however, appears to be unlikely.
In conclusion, we have demonstrated that falcons are highly susceptible to HPAI (H5N1) but can be protected from clinical disease and death by vaccination with a heterologous inactivated vaccine administered intramuscularly or subcutaneously. Virus shedding was grossly reduced after vaccination, thereby decreasing risk for further virus transmission to other avian species as well as to humans. However, use of vaccine will require the establishment of an appropriate surveillance program that includes use of serologic testing, PCR, and sentinel birds.
Dr Lierz is a veterinarian at the Free University of Berlin and is mainly involved in avian and free-ranging animal medicine. His research interests are zoonoses transmitted by birds and free-ranging animals as well as mycoplasmas and the application of new diagnostic techniques to the avian patient.
Acknowledgments
We thank M. Carnarius, A. Kohls, and C. Waldow for support with sampling of the birds; C. Sabel, T. Arnold, and G. Bauer for caring for the birds during vaccination and challenge; and B. Valder, G. Grotehenn, R. Häuslaigner, and R. Wäckerlin for laboratory support.
This study has been partially supported by funds from the Federal Ministry of Food, Agriculture and Consumer Protection (BMELV), Germany (FSI project no. 1-4.1), and the German Falconry Association (DFO).
References
- Perkins LE, Swayne DE. Comparative susceptibility of selected avian and mammalian species to a Hong Kong-origin H5N1 high-pathogenicity avian influenza virus. Avian Dis. 2003;47:956–67. DOIPubMedGoogle Scholar
- Swayne DE, Halvorson DA. Influenza. In: Saif YM, Barnes HJ, Glisson JR, Fadly AM, McDougald LR, Swayne DE, editors. Diseases of poultry, 11th ed. Ames (IA): Iowa State Press; 2003; 135–60.
- Temple SA. Do predators always capture substandard individuals disproportionately from prey populations? Ecology. 1987;68:669–74. DOIGoogle Scholar
- Kuiken T, Rimmelzwaan G, van Riel D, van Amerongen G, Baars M, Fouchier R, Avian H5N1 influenza in cats. Science. 2004;306:241. DOIPubMedGoogle Scholar
- Manvell RJ, McKinney P, Wernery U, Frost K. Isolation of a highly pathogenic influenza A virus of subtype H7N3 from a peregrine falcon. Avian Pathol. 2000;29:635–7. DOIPubMedGoogle Scholar
- Magnino S, Fabbi M, Moreno A, Sala G, Lavazza A, Ghelfi E, Avian influenza virus (H7 serotype) in a saker falcon in Italy. Vet Rec. 2000;146:740.PubMedGoogle Scholar
- Van Borm S, Thomas I, Hanquet G, Lambrecht B, Boschmans M, Dupont G, Highly pathogenic H5N1 influenza virus in smuggled Thai eagles, Belgium. Emerg Infect Dis. 2005;11:702–5.PubMedGoogle Scholar
- Samour J. Avian influenza in Saudi falcons. Falco Newsletter. 2006;27:21 [cited 2007 Aug 30]. Available from http://www.savethesaker.com/images/falco%2027.pdf
- Friedrich-Loeffler Institute. Epidemiology bulletin no. 37/2006; 2006 [in German; cited 2007 Aug 27]. Available from http://www.fli.bund.de/fileadmin/user_upload/dokumente/news/aktuelle_krankheitsgeschehen/avi_flu/lb_influenza060703.pdf
- Subbarao K, Klimov A, Katz J, Regnery H, Lim W, Hall H, Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science. 1998;279:393–6. DOIPubMedGoogle Scholar
- Yuen KY, Chan PKS, Peiris M, Tsang DNC, Que TL, Shortridge KF, Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet. 1998;351:467–71. DOIPubMedGoogle Scholar
- Olsen B, Munster VJ, Wallenstein A, Waldenström J, Osterhaus ADME, Fouchier RAM. Global patterns of Influenza A virus in wild birds. Science. 2006;312:384–8. DOIPubMedGoogle Scholar
- Garcia A, Johnson H, Srivastava DK, Jayawardene DA, Wehr DR, Webster RG. Efficacy of inactivated H5N2 influenza vaccines against lethal A/Chicken/Queretaro/19/95 infection. Avian Dis. 1998;42:248–56. DOIPubMedGoogle Scholar
- Swayne DE, Beck JR, Perdue ML, Beard CW. Efficacy of vaccines in chickens against highly pathogenic Hong Kong H5N1 avian influenza. Avian Dis. 2001;45:355–65. DOIPubMedGoogle Scholar
- Ellis TM, Leung CY, Chow MK, Bissett LA, Wong W, Guan Y, Vaccination of chickens against H5N1 avian influenza in the face of an outbreak interrupts virus transmission. Avian Pathol. 2004;33:405–12. DOIPubMedGoogle Scholar
- Ellis TM, Sims LD, Wong HK, Wong CW, Dyrting KC, Chow KW, Use of avian influenza vaccination in Hong Kong. Dev Biol (Basel). 2006;124:133–43.PubMedGoogle Scholar
- Capua I, Terregino C, Cattoli G, Mulinelli F, Rodriguez JF. Development of a DIVA (Differentiating Infected from Vaccinated Animals) strategy using a vaccine containing a heterologous neuramidase for the control of avian influenza. Avian Pathol. 2003;32:47–55. DOIPubMedGoogle Scholar
- Philippa J, Baas C, Beyer W, Bestebroer T, Fouchier R, Smith D, Vaccination against highly pathogenic avian influenza H5N1 virus in zoos using an adjuvanted inactivated H5N2 vaccine. Vaccine. 2007;25:3800–8.PubMedGoogle Scholar
- Bertelsen MF, Klausen J, Holm E, Grondahl C, Jorgensen PH. Serological response to vaccination against avian influenza in zoo-birds using an inactivated H5N9 vaccine. Vaccine. 2007;25:4345–9. DOIPubMedGoogle Scholar
- Oh S, Martelli P, Hock OS, Luz S, Furley C, Chiek EJ, Field study on the use of inactivated H5N2 vaccine in avian species. Vet Rec. 2005;157:299–300.PubMedGoogle Scholar
- Obon E, Kent J, Bailey T, Donovan DO, McKeown S, Joseph S, Preliminary results of a field trial using the H5N2 avian influenza vaccine in zoological collections in Dubai. Wildlife Middle East Newsletter. 2006;1:4 [cited 2007 Aug 27]. Available from http://www.wmenews.com/newsletters/vol1_issue1/wme_v1i1_enl.pdf
- Heidenreich M. Birds of prey: medicine and management. London: Blackwell Science Ltd.; 1997. p. 35–42, 123
- World Organization for Animal Health. Manual of diagnostic tests for terrestrial animals, 5th edition. 2004 [cited 2007 Aug 27]. Available from http://www.oie.int/eng/normes/mmanual/a_00037.htm
- Spackman E, Senne DA, Myers TJ, Bulaga LL, Garber LP, Perdue ML, Development of a real-time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. J Clin Microbiol. 2002;40:3256–60. DOIPubMedGoogle Scholar
- Weber S, Harder T, Starick E, Beer M, Werner O, Hoffmann B, Molecular analysis of highly pathogenic avian influenza virus of subtype H5N1 isolated from wild birds and mammals in northern Germany. J Gen Virol. 2007;88:554–8. DOIPubMedGoogle Scholar
- Commission of the European Communities. Commission decision of 4 August 2006 (2006/437/EC) approving a diagnostic manual for avian influenza as provided for in Council Directive 2005/94/EC [cited 2007 Aug 27]. Available from http://eur-lex.europa.eu/lexuriserv/site/en/oj/2006/l_237/l_23720060831en00010027.pdf
- Hoffmann B, Depner K, Schirrmeier H, Beer M. A universal heterologous internal control system for duplex real-time RT-PCR assays used in a detection system for pestiviruses. J Virol Methods. 2006;136:200–9. DOIPubMedGoogle Scholar
- Werner O, Starick E, Grund CH. Isolation and characterization of a low-pathogenicity H7N7 influenza virus from a turkey in a small mixed free-range poultry flock in Germany. Avian Dis. 2003;47:1104–6. DOIPubMedGoogle Scholar
- Hoffmann B, Harder T, Starick E, Depner K, Werner O, Beer M. Rapid and highly sensitive pathotyping of avian influenza A H5N1 virus by using real-time reverse transcription-PCR. J Clin Microbiol. 2007;45:600–3. DOIPubMedGoogle Scholar
- Klopfleisch R, Werner O, Mundt E, Harder T, Teifke JP. Neurotropism of highly pathogenic avian influenza virus A/chicken/Indonesia/2003 (H5N1) in experimentally infected pigeons (Columbia livia f. domestica). Vet Pathol. 2006;43:463–70. DOIPubMedGoogle Scholar
- Teifke JP, Klopfleisch R, Globig A, Starick E, Hoffmann B, Wolf PU, Pathology of natural infections by H5N1 highly pathogenic avian influenza virus in mute (Cygnus olor) and whooper (Cygnus cygnus) swans. Vet Pathol. 2007;44:137–43. DOIPubMedGoogle Scholar
- Perkins LE, Swayne DE. Varied pathogenicity of a Hong Kong–origin H5N1 avian influenza virus in four passerine species and budgerigars. Vet Pathol. 2003;40:14–24. DOIPubMedGoogle Scholar
- Perkins LE, Swayne DE. Pathogenicity of a Hong Kong-origin H5N1 highly pathogenic avian influenza virus for emus, geese, ducks and pigeons. Avian Dis. 2002;46:53–63. DOIPubMedGoogle Scholar
- van der Goot JA, Koch G, de Jong MC, van Boven M. Quantification of the effect of vaccination on transmission of avian influenza (H7N7) in chickens. Proc Natl Acad Sci U S A. 2005;102:18141–6. DOIPubMedGoogle Scholar
- Veits J, Wiesner D, Fuchs W, Hoffmann B, Granzow H, Starick E, Newcastle disease virus expressing H5 hemagglutinin gene protects chickens against Newcastle disease and avian influenza. Proc Natl Acad Sci U S A. 2006;103:8197–202. DOIPubMedGoogle Scholar
- Mackay IM, Arden KE, Nitsche A. Real-time PCR in virology. Nucleic Acids Res. 2002;30:1292–305. DOIPubMedGoogle Scholar
- Cinatl J Jr, Michaelis M, Doerr HW. The threat of avian influenza a (H5N1): part II: clues to pathogenicity and pathology. Med Microbiol Immunol (Berl). 2007;196:191–201. DOIGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 13, Number 11—November 2007
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Michael Lierz, Institute for Poultry Diseases, Free University of Berlin, Königsweg 63, 14163 Berlin, Germany;
Top