Volume 13, Number 6—June 2007
Research
Strategies to Reduce Person-to-Person Transmission during Widespread Escherichia coli O157:H7 Outbreak
Cite This Article
Citation for Media
Abstract
During the Escherichia coli O157:H7 outbreak in 2006 in the United States, the primary strategy to prevent illness was to advise consumers not to eat spinach. No widespread warnings were issued about preventing person-to-person (secondary) transmission. A disease transmission model, fitted to the current data, was used to investigate likely reductions in illnesses that could result from interventions to prevent secondary transmission. The model indicates that exposure to contaminated spinach occurred early in the outbreak and that the secondary illness transmission was similar to that in previous E. coli outbreaks (≈12%). The model also suggests that even a modestly effective strategy to interrupt secondary transmission (prevention of only 2%–3% of secondary illnesses) could result in a reduction of ≈5%–11% of symptomatic cases. This analysis supports the use of widespread public health messages during outbreaks of E. coli O157:H7 with specific advice on how to interrupt secondary transmission.
Widespread distribution of contaminated spinach was implicated in an Escherichia coli O157:H7 (E. coli O157) outbreak in the United States in 2006. As of September 24, 2006, a total of 173 cases had been reported in 25 states; 88% of cases were reported over an 18-day period from August 19 through September 5, 2006 (1). The outbreak strain was particularly virulent, resulting in 1 death, 53% of patients being hospitalized, and a 16% rate of hemolytic uremic syndrome. At the time of our analysis, the potential extent of the outbreak was unknown because new cases were still being reported. The Centers for Disease Control and Prevention (CDC) and the US Food and Drug Administration advised consumers not to eat spinach as the primary strategy for protecting against foodborne transmission of E. coli O157 (2). No warnings, however, were issued regarding the prevention of person-to-person (secondary) transmission.
According to recent studies on the extent of secondary transmission for E. coli O157 and other pathogens, the initially reported foodborne illnesses in the outbreak may have represented only a small fraction of a larger outbreak that included asymptomatic infections and secondary infections among household members of infected persons and other close contacts. Specifically, the E. coli O157 literature indicates that a large proportion (72%) of infections are asymptomatic (3), exposure to low doses can result in infection (4), and reported secondary transmission rates are on the order of 4%–16% (5). Further, outbreaks of shigellosis (6), cryptosporidiosis (7), and giardiasis (8,9) indicate that other highly infectious enteric pathogens can spread from person to person after being introduced into a community through water, food, or other sources (9). Recent adenovirus outbreak data indicate that persons with asymptomatic infection who are shedding virus can be a primary cause of continual transmission of infection (10). And in a prolonged giardiasis outbreak that occurred in late 2003 in a Boston, Massachusetts, suburb, 30 primary cases of giardiasis attributed to a children’s swimming pool resulted in 105 secondary cases among persons from the same or socially related households. New cases of giardiasis continued to occur for up to 4 months after the pool was closed for the season (9).
Using an epidemiologically based disease transmission model, we investigated the potential for reducing the number of symptomatic infections (cases) of E. coli O157 by using interventions designed to reduce secondary transmission during the course of the 2006 E. coli O157 outbreak in the United States. We assumed that a combination of possible intervention strategies to interrupt secondary transmission would have a range of possible levels of effectiveness. These strategies would include strongly recommending handwashing, avoiding contact with persons with diarrhea (of any cause), meticulously preparing food, and avoiding work or school when ill with any gastrointestinal sign or symptom. Initiation of these strategies was assumed to occur at the same time as CDC’s first press release on the outbreak on September 14, 2006, 1 week later, and 2 weeks later. We assumed that these strategies would reduce the transmission of infection to healthy persons from persons with both symptomatic and asymptomatic infections.
An existing epidemiologic model for disease transmission (11,12) was adapted to simulate the 2006 US E. coli O157 outbreak. The most recent data from CDC (13) were used, along with data from the published literature, to replicate the E. coli O157:H7 prevalence estimated by CDC in the United States and the reported outbreak conditions as of September 19, 2006 (13), and then to evaluate the potential effect of the timing and effectiveness of interventions on secondary infections.
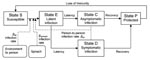
Our modeling approach is consistent with a large base of literature that describes the use of dynamic population models in the study of epidemics (14–16) and environmental disease processes (17–19). Our model consists of 5 population-level epidemiologic states that account for persons who are susceptible (S), exposed (E), infected but asymptomatic carriers (C), diseased (D), and post-infection (P) (Figure 1).
There are 3 possible routes of exposure that move persons from the susceptible (S) to the exposed (E) state. These include environment-to-person infection (βep), person-to-person infection (βpp), and infection through consumption of contaminated spinach (βspinach). We assume that all infections take some time to manifest signs or symptoms (the incubation period). During this incubation period, persons are in the exposed state. Once the infection is clinically apparent, persons are either carriers (in state C) or diseased (D). A proportion of the exposed persons move to the carrier state, C, at a rate inversely related to the duration of incubation (20,21). Symptoms develop in the remaining proportion of the exposed population that becomes infected, and these persons move to the diseased state, D (3). From both the asymptomatic and diseased states, C and D, persons recover and move to state P at a rate inversely proportional to the duration of infection (22–24). Finally, persons in state P become susceptible again, moving to a susceptible state, S, at a rate inversely proportional to the duration of immunity.
The model was first calibrated to CDC’s estimate of 73,480 annual US cases (25) and to reported rates of secondary transmission (5,9). With the model calibrated to transmission levels for endemic E. coli O157, we modeled the additional contribution of cases attributable to the outbreak. We assumed that exposure to contaminated spinach began on August 19, approximately when cases were first identified, and allowed the rate of transmission, (βspinach), and the number of days of exposure to vary to fit the reported outbreak incidence of 131 cases from August 1 through September 19 with 122 of these cases (93%) occurring from August 19 through September 5 (13). The mathematical details of the model and its calibration are described in the online Appendix (available from www.cdc.gov/EID/content13/6/zzz_onlineapp.htm).
The effect of the timing of interventions on person-to-person transmission with various levels of effectiveness was then evaluated. Three timings for the intervention were considered. The first was assumed to be initiated when CDC issued its first press release on the outbreak on September 14, 2006; the second and third timings were 1 and 2 weeks later, respectively. The interventions were assumed to reduce secondary transmission by 1%–25%. The number of averted cases was computed by comparing the number of cases with and without the intervention.
We also considered a range of possible levels of secondary transmission on the basis of prior reports that person-to-person transmission is responsible for 12% (5) and 75% (9) of the cases in a community during an outbreak from a highly infectious and transmissible pathogen. Intermediate values of secondary transmission, 25% and 50%, were also considered. Confidence intervals (CIs) for the number of averted cases due to the interventions were computed from 500 Monte Carlo simulations for the various secondary transmission rates. For each Monte Carlo simulation, we randomly sampled parameter values from the uncertainty ranges specified in the Table. Model simulations were implemented in Mathcad 13.0 (26).
To replicate the relatively large percentage (122 [93%]) of the 131 cases reported from August 19 through September 5, 2006, we assumed that exposure to contaminated spinach likely occurred comparatively early in the outbreak. Fitting the model to the case data was only possible when foodborne exposure occurred before August 22. This date is considerably earlier than CDC’s initial press announcement (27), which was released 23 days later. If substantial foodborne transmission occurred after August 22, secondary infections would have greatly extended the outbreak period. Moreover, 4 days of exposure was sufficient to result in the outbreak, which suggests that exposures were largely limited to the earliest days of the outbreak. Generally, there was also a narrow window in which the outbreak could have started. For instance, we could also fit the calibration data if we assumed the outbreak started a day earlier, on August 18, with 1–4 days of ensuing exposure.
We were able to replicate the case data on the 131 cases with our model under the assumption of 12% secondary infections. However, we could not replicate the case data under the assumption of 50% and 75% secondary infections. For both the 50% and 75% secondary infection assumptions, with a very short period of exposure, it was possible to fit 122 new cases of illness between August 19 and September 5; however, simulations of the model resulted in more secondary infections than suggested by the case data. Under the assumption of 25% secondary infections, the model fit the case data well, with only 5 more new infections after September 5 than reported. This result suggests that either secondary transmission was not as high as 50%–75% for this outbreak or the percentage of cases after September 5 was underreported.
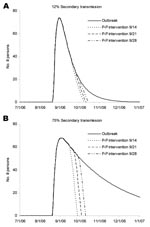
Simulated interventions for person-to-person transmission decreased the number of ill persons, with greater reductions for higher levels of secondary transmission (Figure 2). With higher levels of secondary transmission (Figure 2B), the timing of interventions has a greater effect on the number of averted cases. However, with greater secondary transmission, the outbreak period is extended, which allows more time to organize a campaign against person-to-person transmission and a greater opportunity to reduce secondary hospitalizations and deaths.
Considering the number of cases that could be averted by an intervention such as a campaign to encourage handwashing and isolation of persons with diarrhea under a varying range of effectiveness (Figure 3), we found that such programs can be inefficient and still substantially reduce secondary transmission. Even if a campaign were initiated relatively late in the outbreak (day 51), the number of cases would be reduced. The reduction increases exponentially with increasing levels of secondary transmission (Figure 3). Specifically, with 12% secondary transmission, ≈6 (5%) of cases are averted. Averted cases increase to 16 (11%) and 61 (29%) with 25% and 50% secondary transmission, respectively. With 75% secondary transmission, a much larger number of illnesses can be averted (≈225 [57%]). The 95% CIs for the 12% secondary transmission rate scenario were 0.2–19 cases averted (0%–14% of total illnesses); for the 25% secondary transmission rate scenario, CI were 0.5–59 cases averted (0%–38% of total cases).
The first mathematical models to analyze the spread and control of infectious diseases were developed in the early 20th century in attempts to understand measles (28) and malaria (29). This field grew exponentially in the middle of the 20th century. A tremendous variety of models have now been formulated, mathematically analyzed, and applied to infectious diseases (16). Mathematical models of disease transmission have become important tools that have led to understanding the transmission characteristics of infectious diseases in communities and better approaches to decreasing the transmission of these diseases (16,30). We applied such a model to the 2006 spinach-associated E. coli O175 outbreak to analyze data as they were still being collected to evaluate the effectiveness of strategies that might reduce person-to-person transmission of infection. The model as constructed allows for investigation across the full range of possible values for all relevant variables, including the rate of secondary transmission and the effectiveness of intervention strategies.
Public health messages about the importance of disease prevention methods (such as frequent handwashing, covering one’s mouth when coughing or sneezing, and staying at home when ill) to prevent secondary transmission of infection routinely are conveyed during influenza outbreaks (31). Although the prevention of secondary transmission is a specific goal of hospital guidelines for the care of patients with diarrhea, such advice was not a focus of the public health messages disseminated for the 2006 E. coli O157 outbreak. We hypothesized that the interruption of secondary transmission might have had a useful role as an additional tool in managing this outbreak. A mathematical model that replicated the published data available as of September 19, 2006, was created. This model was used to examine the effect that various levels of effective interruption of secondary transmission would have on the course of the outbreak.
The model results suggested 2 findings. First, exposure to contaminated spinach apparently occurred early in the outbreak and was likely at low levels after that. Second, this E. coli outbreak appears to be more similar to previous E. coli outbreaks than to a large-scale 2003 giardiasis outbreak (8,9), in terms of person-to-person contribution to the overall outbreak-attributable incidence of infection. Despite wide confidence bounds on our estimates, our findings suggest that even a modestly effective strategy to interrupt secondary transmission (resulting in prevention of 2%–3% of secondary cases) could have resulted in a median reduction of ≈5%–11% of infected and symptomatic persons. Not all secondary infections are averted by the interventions because they are assumed to be initiated relatively late and because they are not completely effective. The number of averted cases, however, increases exponentially with increasing rates of secondary transmission, and the results suggest that these programs would have substantial benefits even if they are implemented relatively late in an outbreak.
Limitations
Several simplifying assumptions were needed to conduct the analysis. These assumptions relate to the form of the disease transmission model, interpretation of the available outbreak data, and treatment of the variability and uncertainty in the data used to inform the model.
With respect to the selected disease transmission model, a variety of model forms can be used to characterize infectious disease transmission and to evaluate the potential for effective interventions. Particular characteristics of each model form capture different aspects of the disease transmission system (32). In this analysis, the salient assumption was that the epidemiologic status of the population could be approximated reasonably well with the relatively simple structure of the disease transmission model. Other model structures also might yield additional or alternative insights. For example, if “super-spread” events are important determinants in characterizing the magnitude of disease transmission (30) during outbreaks such as this one, stochastic dynamic modeling may be necessary.
Another limitation of our study is the lack of data on efficacy of person-to-person reduction strategies specific for E. coli transmission. The person-to-person transmission rate and reduction due to handwashing were assumed to be the same for both symptomatic and asymptomatic persons, and the effect on the course of the epidemic (specifically, the number of cases averted) of these strategies was examined for a wide range of possible levels of effectiveness. Symptomatic and asymptomatic persons were assumed to transmit E. coli O:157 to susceptible persons in the same manner, since we have no data to suggest otherwise. It is possible that symptomatic-to-susceptible transmission may be greater than asymptomatic-to-susceptible transmission and that interventions may be more effective among persons with symptoms. Increasing the rate of symptomatic-to-susceptible transmission by 8% over the asymptomatic-to-susceptible rate indeed results in a small increase in averted cases. If new data on the differences between person-to-person transmission rates and/or person-to-person reduction efficacies become available, the parameterization of the model could be improved.
As noted previously, detailed information describing the timing of exposures to E. coli O157 through contaminated spinach and subsequent outbreak cases was not yet available at the time of our analysis. Thus, the model is limited by the precision and completeness of the case-reporting data. The model can be updated easily when additional data become publicly available. Despite these limitations, available data were sufficient to suggest that foodborne transmission was terminated early in the outbreak and that interventions to reduce secondary transmission could be very effective.
Finally, in the interest of producing timely results that might influence the control of the outbreak, median parameter values were used to calibrate exposure to the observed case data. Holding these exposures constant, Monte Carlo simulations were subsequently used to explore the variance and uncertainty in the estimates of averted cases. This resulted in large confidence bounds. When more complete case data become available, the data may reduce the uncertainty not only in the timing of the exposure but also in the values of the remaining parameters and the estimate of averted cases. In the past, we have explored such calibrations with Monte Carlo methods (11,33–35).
Public Health Implications
Public health strategies for preventing secondary transmission could include public media campaigns reminding the population of the importance of handwashing, avoiding contact with feces, minimizing nonessential contact with persons with diarrhea, meticulous care when preparing and consuming food, and staying at home from work or school when having any diarrhea during the outbreak period. Any of these strategies could be targeted to communities in which any cases of E. coli O:157 had been reported and scaled to regional or national audiences when appropriate. Messages for all of these strategies can be delivered inexpensively to large or targeted populations through a variety of media (television, radio, print, Internet). That public health officials already have the ability to launch rapid and successful infection control messages to the public was demonstrated during the outbreak of severe acute respiratory syndrome (36). In the future, public health officials might even rapidly deliver urgent health messages to a large population in a city or region through voluntary preregistration of email addresses as part of an emergency alert network that includes the media and public health officials. At the first appearance of evidence of an E. coli O157 outbreak, a message with clear instructions could be distributed to thousands or tens of thousands people at risk locally, regionally, or nationally, and to specific subgroups at high risk, such as the young, the elderly, or the immunocompromised.
We did not formally estimate in our model the economic tradeoffs between a public health campaign to reduce secondary transmission compared with the costs of hospitalizations and medical care for persons with this disease. However, because the hospitalization costs of a single E. coli O157 case complicated by death from hemolytic uremic syndrome are estimated to be as high as US $6.2 million per case, we believe that such a campaign would be highly cost-effective (37). Given the potential public health benefits to be gained by these actions, and the low costs associated with their implementation, these strategies also may be relevant for outbreaks from other highly infectious pathogenic microorganisms.
The individual effects of these intervention strategies when used alone or in combination to interrupt secondary transmission were not modeled. Rather, we assumed that a combination of strategies would be used and would have some combined benefit. We intentionally examined the possible benefits across a wide range of possible levels of effectiveness. As expected, higher levels of effectiveness resulted in greater impact on the outbreak. However, even fairly low levels of intervention effectiveness (such as 2%–3% interruption in secondary transmission) led to reductions (5%–11%) in the number of cases attributable to the outbreak. Further study is needed select the individual secondary control strategies to use if limiting the number of specific prevention strategies is necessary.
Implications for Future Outbreaks
Public health officials have the necessary authority to issue and widely distribute guidelines for preventing secondary transmission. Some readers might question whether our results are a sufficient demonstration to justify a large-scale campaign by public health officials to prevent secondary transmission. If additional proof were demanded, various study designs that could be used to evaluate the effectiveness of media campaigns to help to control outbreaks. As 1 example, a relatively simple ecologic study could correlate incidence rates with local press coverage in different communities. A more definitive design, however, would be a randomized, controlled trial. Such a trial might, for example, randomize 50% of the affected areas to a “media-blitz” that explained the importance of handwashing, stool precautions, and other measures including those discussed above; the other communities would receive the public health messages currently delivered during outbreaks. The rapidity with which the outbreak is terminated would be the principal outcome of interest. However, obtaining approval to run such an experiment may be difficult because such a trial would only be ethically justified if the investigators could convince a review panel that sufficient uncertainty about the effectiveness of the intervention exists.
Our analysis of the 2006 E. coli O157 outbreak due to contaminated spinach in the United States supports the assertion that health officials should consider rapidly delivering widespread public health messages with specific advice on how to interrupt secondary transmission of E. coli O157. The results suggest that such an intervention, even if only modestly successful, could meaningfully reduce the number of cases.
Dr Seto is a research scientist in the Department of Environmental Health Sciences in the School of Public Health at the University of California, Berkeley. His primary research interests are infectious disease epidemiology, spatial analysis, and mathematical modeling.
Acknowledgment
We thank George Tchobanoglous for his critical review of the article.
References
- Centers for Disease Control and Prevention. Update on multi-state outbreak of E. coli O157:H7 infections from fresh spinach, September 24, 2006. [cited 2006 Oct 2]. Available from http://www.cdc.gov/ecoli/2006/september/updates/092406.htm
- Centers for Disease Control and Prevention. CDC advice for consumers on the multistate outbreak of E. coli from fresh spinach. 2006. [cited 2006 Oct 17]. Available from http://www.cdc.gov/ecoli/2006/september/consumeradvice.htm
- Ludwig K, Sarkim V, Bitzan M, Karmali MA, Bobrowski C, Ruder H, Shiga toxin-producing Escherichia coli infection and antibodies against Stx2 and Stx1 in household contacts of children with enteropathic hemolytic-uremic syndrome. J Clin Microbiol. 2002;40:1773–82. DOIPubMedGoogle Scholar
- Teunis P, Takumi K, Shinagawa K. Dose response for infection by Escherichia coli O157:H7 from outbreak data. Risk Anal. 2004;24:401–7. DOIPubMedGoogle Scholar
- Parry SM, Salmon RL. Sporadic STEC O157 infection: secondary household transmission in Wales. Emerg Infect Dis. 1998;4:657–61. DOIPubMedGoogle Scholar
- Mohle-Boetani JC, Stapleton M, Finger R, Bean NH, Poundstone J, Blake PA, Communitywide shigellosis: control of an outbreak and risk factors in child day-care centers. Am J Public Health. 1995;85:812–6. DOIPubMedGoogle Scholar
- Fox LM, Ocfemia MC, Hunt DC, Blackburn BG, Neises D, Kent WK, Emergency survey methods in acute cryptosporidiosis outbreak. Emerg Infect Dis. 2005;11:729–31.PubMedGoogle Scholar
- Bell BP, Goldoft M, Griffin PM, Davis MA, Gordon DC, Tarr PI, A multistate outbreak of Escherichia coli O157:H7-associated bloody diarrhea and hemolytic uremic syndrome from hamburgers. The Washington experience. JAMA. 1994;272:1349–53. DOIPubMedGoogle Scholar
- Katz DE, Heisey-Grove D, Beach M, Dicker RC, Matyas BT. Prolonged outbreak of giardiasis with two modes of transmission. Epidemiol Infect. 2006;134:935–41. DOIPubMedGoogle Scholar
- Russell KL, Broderick MP, Franklin SE, Blyn LB, Freed NE, Moradi E, Transmission dynamics and prospective environmental sampling of adenovirus in a military recruit setting. J Infect Dis. 2006;194:877–85. DOIPubMedGoogle Scholar
- Eisenberg JN, Seto EYW, Olivieri AW, Spear RC. Quantifying water pathogen risk in an epidemiological framework. Risk Anal. 1996;16:549–63. DOIPubMedGoogle Scholar
- Soller JA, Eisenberg J, DeGeorge J, Cooper R, Tchobanoglous G, Olivieri A. A public health evaluation of recreational water impairment. J Water Health. 2006;4:1–19.PubMedGoogle Scholar
- Centers for Disease Control and Prevention. Update on multi-state outbreak of E. coli O157:H7 infections from fresh spinach, September 19, 2006. [cited 2006 Sep 21]. Available from http://www.cdc.gov/ecoli/2006/september/updates/091906.htm
- Anderson RM, May R. Infectious diseases of humans: dynamics and control. New York: Oxford University Press; 1991.
- Hethcote H. Qualitative analyses of communicable disease models. Math Biosci. 1976;28:335–56. DOIGoogle Scholar
- Hethcote HW. The mathematics of infectious diseases. SIAM Rev. 2000;42:599–653 http://epubs.siam.org/SIREV/sirev_toc.html. DOIGoogle Scholar
- Koopman J. Modeling infection transmission. Annu Rev Public Health. 2004;25:303–26. DOIPubMedGoogle Scholar
- Koopman JS, Jacquez G, Chick SE. New data and tools for integrating discrete and continuous population modeling strategies. Ann N Y Acad Sci. 2001;954:268–94. DOIPubMedGoogle Scholar
- Koopman JS, Longini IM, Jacquez JA, Simon CP. Assessing risk factors for transmission of infection. Am J Epidemiol. 1991;133:1199–209.PubMedGoogle Scholar
- Boyce TG, Swerdlow DL, Griffin PM. Escherichia coli O157:H7 and the hemolytic-uremic syndrome. N Engl J Med. 1995;333:364–8. DOIPubMedGoogle Scholar
- Nataro JP, Kaper JB. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201.PubMedGoogle Scholar
- Belongia EA, Osterholm MT, Soler JT, Ammend DA, Braun JE, MacDonald KL. Transmission of Escherichia coli O157:H7 infection in Minnesota child day-care facilities. JAMA. 1993;269:883–8. DOIPubMedGoogle Scholar
- Shah S, Hoffman R, Shillam P, Wilson B. Prolonged fecal shedding of Escherichia coli O157:H7 during an outbreak at a day care center. Clin Infect Dis. 1996;23:835–6. DOIPubMedGoogle Scholar
- Swerdlow DL, Griffin PM. Duration of faecal shedding of Escherichia coli O157:H7 among children in day-care centres. Lancet. 1997;349:745–6. DOIPubMedGoogle Scholar
- Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, Food-related illness and death in the United States. Emerg Infect Dis. 1999;5:607–25. DOIPubMedGoogle Scholar
- Mathsoft Engineering & Education, Inc. Mathcad v.13. Cambridge (MA): Mathsoft Engineering & Education, Inc; 2005.
- Centers for Disease Control and Prevention. Multiple states investigating a large outbreak of E. coli O157:H7 infections, September 14, 2006. [cited 2006 Sep 21]. Available from http://www2a.cdc.gov/HAN/ArchiveSys/ViewMsgV.asp?AlertNum=00249
- Hamer WH. Endemic disease in England. Lancet. 1906;1:733–9.
- Ross R. The prevention of malaria. 2nd ed. London: Murray; 1911.
- Riley S, Fraser C, Donnelly CA, Ghani AC, Abu-Raddad LJ, Hedley AJ, Transmission dynamics of the etiological agent of SARS in Hong Kong: impact of public health interventions. Science. 2003;300:1961–6. DOIPubMedGoogle Scholar
- Smith NM, Bresee JS, Shay DK, Uyeki TM, Cox NJ, Strikas RA. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2006;55(RR-10):1–42.PubMedGoogle Scholar
- Soller JA. Use of microbial risk assessment to inform the national estimate of waterborne illness from drinking water. J Water Health. 2006;4(Suppl 2):165–86. DOIPubMedGoogle Scholar
- Eisenberg JNS, McKone TE. Decision tree method for the classification of chemical pollutants: incorporation of across-chemical variability and within-chemical uncertainty. Environ Sci Technol. 1998;32:3396–404. DOIGoogle Scholar
- Soller JA, Olivieri A, Crook J, Parkin R, Spear R, Tchobanoglous G, Risk-based approach to evaluate the public health benefit of additional wastewater treatment. Environ Sci Technol. 2003;37:1882–91. DOIPubMedGoogle Scholar
- Spear RC, Hubbard A, Liang S, Seto E. Disease transmission models for public health decision making: toward an approach for designing intervention strategies for Schistosomiasis japonica. Environ Health Perspect. 2002;110:907–15. DOIPubMedGoogle Scholar
- Posid JM, Bruce SM, Guarnizo JT, Taylor ML, Garza BW. SARS: mobilizing and maintaining a public health emergency response. J Public Health Manag Pract. 2005;11:208–15.PubMedGoogle Scholar
- Frenzen PD, Drake A, Angulo FJ. Emerging Infections Program FoodNet Working Group. Economic cost of illness due to Escherichia coli O157 infections in the United States. J Food Prot. 2005;68:2623–30.PubMedGoogle Scholar
Figures
Table
Cite This ArticleTable of Contents – Volume 13, Number 6—June 2007
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Jeffrey Soller, Soller Environmental, 3022 King St, Berkeley, CA 94703, USA
Top