Volume 13, Number 9—September 2007
Letter
Highly Pathogenic Porcine Reproductive and Respiratory Syndrome, China
Cite This Article
Citation for Media
To the Editor: Since April 2006, a highly pathogenic disease caused by unknown agents and characterized by high fever and a high proportion of deaths in pigs of all ages, emerged in some swine farms in Jiangxi Province, People’s Republic of China. The morbidity rate was 50%–100% and mortality rate was 20%–100%. In the next several months, the disease spread rapidly to most provinces of China. In almost all affected swine herds, the following clinical signs were observed: high and continuous fever, anorexia, red discolorations in the bodies, and blue ears; in the late phase of the disease, diarrhea and other clinical signs might be seen due to the secondary infections. Clinical samples (from lungs, kidneys, liver, and lymph nodes) were collected from animals in different provinces and sent for laboratory diagnosis. DNA and RNA were extracted from the tissue homogenate and PCR or reverse transcription–PCR (RT-PCR) was conducted to detect porcine reproductive and respiratory syndrome virus (PRRSV), classic swine fever virus, porcine circovirus, and pseudorabies virus, respectively (1). In clinical samples, only PRRSV was found to be the dominant virus (48 of 50 samples were PRRSV positive). PRRSVs were then isolated successfully on MARC-145 cells with an obvious cytopathologic effect, characterized by cell congregation, contraction, and brushing off at passage 2; immunofluorescence assay using PRRSV NP-, M- and GP5-specific monoclonal antibodies confirmed that the isolated viruses were PRRSV (2,3). Full-length genomic sequencing of 1 of the isolates (HuN4 strain) showed extensive amino acid (aa) mutations in GP5 protein and 2 deletions in Nsp2, 1 aa deletion at 482, and 29 aa deletions at 533–561, compared with the previous Chinese isolates CH-1a and BJ-4.
The newly isolated PRRSV was used to examine the pathogenicity in 60-day-old PRRSV-free piglets, under closed and biosafety (P2) conditions. Each of the piglets (N = 5) received intranasally 105.0 50% tissue culture infecting dose of the isolated virus propagated in MARC-145 cells (4,5). The animals were kept in separate rooms throughout the experiment. Clinical observations of respiratory signs, behavior, rectal temperature, and coughing were recorded daily. Blood samples were collected every 2 days and tested for PRRSV-specific antibodies by ELISA (6,7). Tissue samples (from heart, lungs, kidneys, spleen, and lymph nodes) from all animals that died during the experiment were collected and detected by histopathologic examination (8) and virus isolation. Results showed that the clinical manifestations of all pigs were similar to those that appeared in the field investigation (including high and continuous fever, anorexia, red discolorations in the bodies, and blue ears). The specific antibodies to PRRSV were detected at 8 days postinfection, and the high antibody level lasted until the animal’s death, and all infected pigs died at either 7, 8, 12, 16, or 21 days postinoculation, respectively. Furthermore, viruses reisolated from the dead pigs showed an identical homology with the inoculated PRRSV in genes coding for GP5 and partial Nsp2 (2,535–3,307 nt). The results showed that the emerging PRRSV, characterized by deletions in Nsp2, is highly pathogenic to pigs.
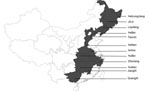
To investigate whether the emerging PRRSV was the causative agent of the pandemic diseases on swine farms, an extensive virus survey was conducted. More than 48 samples collected from different swine farms in12 provinces were found to be PRRSV positive by RT-PCR, based on open reading frame (ORF) 5 and Nsp2 (Figure). Sequence analysis of ORF5 and partial Nsp2 showed that these PRRSVs are highly homologous to each other (98.5%–100% for GP5; 98.2%–100% for Nsp2) and share the same deletions at the same positions of Nsp2 gene with HuN4 strain. Sequence comparison of ORF5 indicated that the HuN4 strain shares 93%, 86%, and 88% nucleotide identities with CH-1a (Chinese isolate), BJ-4 (Chinese isolate), and VR2332 (American isolate), respectively. All the newly isolated PRRSVs belong to the North American type.
Although the cause of the emerging pandemic disease of pigs with a high proportion of deaths in 2006 is unknown, we found high correlation between PRRSV isolation rate and the diseased pigs. The regression test in its natural animal showed that the newly isolated PRRSV was much more virulent than earlier PRRSV isolates. Also, sequence analysis demonstrated a substantial diversity from the PRRSVs isolated during 1996–2005. Further study is needed to answer the question: What role did the newly isolated PRRSV play in the 2006 outbreaks on many of the swine farms in China?
Acknowledgment
The study was supported by grants from the National Basic Research Program (973 plan) of China (no. 2005CB523200), National Scientific Supporting Program (no. 2006BAD06A03/01/04), and National Science Foundation of China (no. 30470072).
References
- Larochelle R, Magar R. Evaluation of the presence of porcine reproductive and respiratory syndrome virus in packaged pig meat using virus isolation and polymerase chain reaction (PCR) method. Vet Microbiol. 1997;58:1–8. DOIPubMedGoogle Scholar
- Nelson EA, Christopher-Hennings J, Drew T, Wensvoort G, Collins JE, Benfield DA. Differentiation of U.S. and European isolates of porcine reproductive and respiratory syndrome virus by monoclonal antibodies. J Clin Microbiol. 1993;31:3184–9.PubMedGoogle Scholar
- Yoon IJ, Joo HS, Christianson WT, Kim HS, Collins JE, Morrison RB, An indirect fluorescent antibody test for the detection of antibody to swine infertility and respiratory syndrome virus in swine sera. J Vet Diagn Invest. 1992;4:144–7.PubMedGoogle Scholar
- Lopez Fuertes L, Domenech N, Alvarez B, Ezquerra A, Dominguez J, Castro JM, Analysis of cellular immune response in pigs recovered from porcine respiratory and reproductive syndrome infection. Virus Res. 1999;64:33–42. DOIPubMedGoogle Scholar
- Horter DC, Pogranichniy RM, Chang CC, Evans RB, Yoon KJ, Zimmerman JJ. Characterization of the carrier state in porcine reproductive and respiratory syndrome virus infection. Vet Microbiol. 2002;86:213–8. DOIPubMedGoogle Scholar
- Albina E, Piriou L, Hutet E, Cariolet R, Hospitalier RL. Immune responses in pigs infected with porcine reproductive and respiratory syndrome virus (PRRSV). Vet Immunol Immunopathol. 1998;61:49–66. DOIPubMedGoogle Scholar
- Johnson W, Roof M, Vaughn E, Christopher-Hennings J, Johnson CR, Murtaugh MP. Pathogenic and humoral immune responses to porcine reproductive and respiratory syndrome virus (PRRSV) are related to viral load in acute infection. Vet Immunol Immunopathol. 2004;102:233–47. DOIPubMedGoogle Scholar
- Nielsen J, Botner A, Bille-Hansen V, Oleksiewicz MB, Storgaard T. Experimental inoculation of late term pregnant sows with a field isolate of porcine reproductive and respiratory syndrome vaccine-derived virus. Vet Microbiol. 2002;84:1–13. DOIPubMedGoogle Scholar
Figure
Cite This ArticleRelated Links
Table of Contents – Volume 13, Number 9—September 2007
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Guang-Zhi Tong, National Key Laboratory of Veterinary Biotechnology, Harbin Veterinary Research Institute, CAAS, no. 427 Maduan St, Harbin 150001, People’s Republic of China;
Top