Volume 14, Number 12—December 2008
Dispatch
Rickettsia parkeri in Argentina
Cite This Article
Citation for Media
Abstract
Clinical reports of an eschar-associated rickettsiosis in the Paraná River Delta of Argentina prompted an evaluation of Amblyomma triste ticks in this region. When evaluated by PCR, 17 (7.6%) of 223 questing adult A. triste ticks, collected from 2 sites in the lower Paraná River Delta, contained DNA of Rickettsia parkeri.
Argentina is a large, ecologically diverse country, with at least 10 Neotropical Amblyomma tick species that bite humans, including Amblyomma triste (1,2; Figure 1, panels A, B). Spotted fever group rickettsiae have been identified in 3 Amblyomma species in Argentina: Rickettsia amblyommii and R. bellii in A. neumanni ticks from Córdoba Province (3), a novel Rickettsia sp. in A. parvum ticks from Córdoba Province (4), and R. rickettsii and R. bellii in A. cajennense ticks from Jujuy Province (5). Human diseases in Argentina attributable to tick-borne rickettsiae have been recognized only recently, including several fatal cases of Rocky Mountain spotted fever caused by R. rickettsii in Jujuy Province (5,6), and a milder, eschar-associated, spotted fever rickettsiosis in the Paraná River Delta of Buenos Aires Province (7) that closely resembles a newly recognized rickettsial spotted fever in the United States caused by R. parkeri (8). R. parkeri has been detected recently in A. triste ticks collected in Uruguay and Brazil (9,10). We report the occurrence of R. parkeri in A. triste ticks collected along the Paraná River close to the locations of several recently identified cases of eschar-associated spotted fever.
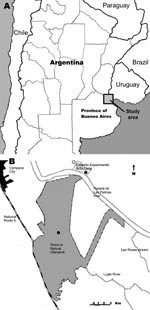
Tick collections occurred at 2 sites in Buenos Aires Province, Argentina, during January through December 2007: Reserva Natural Otamendi (34°15′S, 58°52′W) (Figure 1, panel C) and Estación Experimental, Instituto Nacional de Tecnología Agropecuaria (INTA), Delta del Paraná (34°11′S, 58°50′W) (Figure 1, panel D). Both are located in the lower Paraná River Delta region (Figure 2), which is the southern extension of the Paranense Province of the Amazon Phytogeographic Dominion. The region is characterized by a system of levees that surround temporarily or permanently flooded freshwater marshes (11). Humboldt’s willow (Salix humboldtiana), Cockspur coral tree (Erythrina crista-galli), and Sapium hematospermum grow on the levees, and several species of bulrush (Scirpus giganteus, Schoenoplectus californicus, Scirpus americanus, and Typha sp.) and espadaña (Zizaniopsis bonariensis) comprise the dominant vegetation in the marshes. Medium to large mammals found at the study sites include wild marsh deer (Blastocerus dichotomus), capybara (Hydrochoerus hydrochaeris), pampas fox (Lycalopex gymnocercus), Geoffroy’s cat (Oncifelis geoffroyi), cattle, horses, and dogs.
Questing adult ticks were collected from vegetation on the levees and in the marshes by using cloth flags and preserved in 96% ethanol. All ticks were identified by using standard taxonomic keys (12). A. triste ticks were the only ticks collected from vegetation. For molecular analyses, individual specimens were removed from the ethanol solution, air-dried, and minced with a sterile scalpel blade. DNA was extracted by using a QIAamp DNA Mini-Kit (QIAGEN, Valencia, CA, USA) and eluted in a final volume of 100 μL. DNA extracts were evaluated by using a nested PCR designed to amplify a segment of the rickettsial outer membrane protein A gene (ompA) as described previously (13). In brief, 5 μL of each DNA extract was used as template with primers 190.70 and 190.701 in the primary reaction. Two microliters of each completed primary reaction was used as template with primers 190-FN1 and 190-RN1 in the nested reactions. Primers were used at a final concentration of 300 nmol/L in a 50-μL reaction mixture. All amplicons were sequenced and compared to those in GenBank by using the BLAST 2.0 program (http://blast.ncbi.nlm.nih.gov/blast.cgi). Separate laboratory rooms were used for extracting tick DNA, performing primary and nested PCRs, and sequencing reactions. Water blanks were used for each primary and nested assay, and all extracts that provided amplicons of the expected size were retested to confirm the result.
Amplicons were obtained from DNA extracts of 4 (5.8%) of 69 A. triste ticks collected from Reserva Natural Otamendi and 13 (8.4%) of 154 ticks collected from Estación Experimental INTA Delta del Paraná (Table). All 17 DNA samples produced amplicons of the expected sizes in primary reaction and nested reactions of the assay. Each 590-bp product (excluding primers) from the primary reaction was sequenced, and all sequences showed 100% identity with each other (GenBank accession no. FJ172358) and with the corresponding ompA sequence of R. parkeri (U43802).
This study provides definitive evidence of R. parkeri in Argentina. Our findings have relevance for public health because the infected ticks were collected from the lower Paraná River Delta near the origin of several recently identified cases of eschar-associated rickettsiosis (7; Alfredo Seijo, pers. comm.). In Argentina, at least 15 species of hard ticks bite humans (1); however, the only Amblyomma tick reported to bite humans in the lower Paraná River Delta is A. triste (2). Our data suggest that A. triste ticks are vectors of R. parkeri in this region of Argentina. The prevalence of R. parkeri–infected A. triste ticks identified at these 2 locations is within the range of the infection prevalence of this agent reported in questing adult ticks collected in the state of São Paulo, Brazil (9.7%) and in Canalones County in southern Uruguay (2.6%) (9,10).
In South America, R. parkeri has been detected only in A. triste ticks (9,10), and in the United States, R. parkeri is found almost exclusively in A. maculatum ticks (8,13). A. triste and A. maculatum ticks are phylogenetically and morphologically similar, and R. parkeri appears to be strongly associated with these closely related tick species. Another human-biting Neotropical tick, A. tigrinum, is closely related to A. triste and A. maculatum ticks (12). In this context, A. tigrinum ticks may also be involved in the transmission of R. parkeri in South America. The distribution of A. triste ticks extends from Argentina to Mexico, but this tick has been reported to bite humans only in a few regions of Argentina, Uruguay, and Venezuela (1). Because we are not aware of any records to indicate that immature stages of A. triste ticks will bite humans (1,2,14), our investigation focused on adult questing ticks for evidence of infection with R. parkeri. Preliminary studies indicate that peak adult A. triste abundance and activity in the lower Paraná River Delta occurs during August through November (S. Nava, unpub. data), similar to the seasonal distribution described for A. triste populations in southern Uruguay (14); most cases of eschar-associated disease in Argentina occur during this same interval (7; A. Seijo, pers. comm.).
No immature A. triste ticks were collected by flagging during this investigation. However, larvae and nymphs were found at these study sites attached to the guinea pig (Cavia aperea) amd several species of sigmodontine rodents, including Azara’s grass mouse (Akodon azarae), the yellow pygmy rice rat (Oligoryzomys flavescens), the black-footed pygmy rice rat (O. nigripes), the red hocicudo (Oxymycterus rufus), and the Argentine swamp rat (Scapteromys aquaticus) (S. Nava, unpub. data). These findings suggest that one or more of these species may be involved in the natural transmission cycle of R. parkeri in this region.
Ecologic studies of A. triste ticks collected along the Paraná River in the states of São Paulo and Mato-Grosso do Sul in Brazil indicate that this tick is well-adapted to marsh habitats (15); the results of this investigation support this observation. The occurrence of an R. parkeri rickettsiosis-like disease in humans in the Paraná River Delta suggests that similar cases of human illness may occur in palustrine regions of other Central and South American countries where this tick is found. Additional studies are needed to better understand the natural history of R. parkeri in Argentina and in other countries of the Western Hemisphere.
Dr Nava is a researcher with INTA in Santa Fe, Argentina. His research interests focus on the ecology, systematics, and control of Neotropical ticks.
Acknowledgments
We thank James Gathany for providing the photographs of A. triste, Zoi Antoniadou for assistance with the molecular studies, and Alberto Guglielmone for his critical review of the manuscript.
INTA, Asociación Cooperadora INTA Rafaela, and Consejo Nacional de Investigaciones Científicas y Técnicas provided support to S.N. and M.M.
References
- Guglielmone AA, Beati L, Barros-Battesti DM, Labruna MB, Nava S, Venzal JM, Ticks (Ixodidae) on humans in South America. Exp Appl Acarol. 2006;40:83–100. DOIPubMedGoogle Scholar
- Guglielmone AA, Nava S. Las garrapatas del género Amblyomma como parásitos de humanos en la Argentina. En Temas de Zoonosis III. Buenos Aires: Asociación Argentina de Zoonosis; 2006. p. 269–77.
- Labruna MB, Pacheco RC, Nava S, Brandao PE, Richtzenhain LJ, Guglielmone AA. Infection by Rickettsia bellii and “Rickettsia amblyommii” in Amblyomma neumanni ticks from Argentina. Microb Ecol. 2007;54:126–33. DOIPubMedGoogle Scholar
- Pacheco RC, Moraes-Filho J, Nava S, Brandao PE, Richtzenhain LJ, Labruna MB. Detection of a novel spotted fever group rickettsia in Amblyomma parvum ticks (Acari: Ixodidae) from Argentina. Exp Appl Acarol. 2007;43:63–71. DOIPubMedGoogle Scholar
- Paddock CD, Fernandez S, Echenique GA, Sumner JW, Reeves WK, Zaki SR, Rocky Mountain spotted fever in Argentina. Am J Trop Med Hyg. 2008;78:687–92.PubMedGoogle Scholar
- Ripoll CM, Remondegui CE, Ordoñez G, Arazamendi R, Fusaro H, Hyman MJ, Evidence of rickettsial spotted fever and ehrlichial infections in a subtropical territory of Jujuy, Argentina. Am J Trop Med Hyg. 1999;61:350–4.PubMedGoogle Scholar
- Seijo A, Picollo M, Nicholson WL, Paddock CD. Fiebre manchada por rickettsias en el delta del Parana: una enferedad emergente. Medicina (B Aires). 2007;67:723–6.PubMedGoogle Scholar
- Paddock CD, Finley RW, Wright CS, Robinson HN, Schrodt BJ, Lane CC, Rickettsia parkeri rickettsiosis and its clinical distinction from Rocky Mountain spotted fever. Clin Infect Dis. 2008;47:1188–96. DOIPubMedGoogle Scholar
- Pacheco RC, Venzal JM, Richtzenhain LJ, Labruna MB. Rickettsia parkeri in Uruguay. Emerg Infect Dis. 2006;12:1804–5.PubMedGoogle Scholar
- Silveira I, Pacheco RC, Szabó MP, Ramos HG, Labruna MB. Rickettsia parkeri in Brazil. Emerg Infect Dis. 2007;13:1111–3.PubMedGoogle Scholar
- Kandus P, Malvárez AI. Vegetation patterns and change analysis in the lower delta islands of the Paraná River (Argentina). Wetlands. 2004;24:620–32. DOIGoogle Scholar
- Estrada-Peña A, Venzal JM, Mangold AJ, Cafrune MM, Guglielmone AA. The Amblyomma maculatum Koch, 1844 (Acari: Ixodidae: Amblyomminae) tick group: diagnostic characters, description of the larva of A. parvitarsum Neumann, 1901, 16S rDNA sequences, distribution and hosts. Syst Parasitol. 2005;60:99–112. DOIPubMedGoogle Scholar
- Sumner JW, Durden LA, Goddard J, Stromdahl EY, Clark KL, Reeves WK, Gulf Coast ticks (Amblyomma maculatum) and Rickettsia parkeri, United States. Emerg Infect Dis. 2007;13:751–3.PubMedGoogle Scholar
- Venzal JM, Estrada-Peña A, Castro O, de Souza CG, Félix ML, Nava S, Amblyomma triste Koch 1844 (Acari: Ixodidae): hosts and seasonality of the vector of Rickettsia parkeri in Uruguay. Vet Parasitol. 2008;155:104–9. DOIPubMedGoogle Scholar
- Szabó MP, Castro MB, Ramos HG, Garcia MV, Castagnolli KC, Pinter A, Species diversity and seasonality of free-living ticks (Acari: Ixodidae) in the natural habitat of wild marsh deer (Blastocerus dichotomus) in Southeastern Brazil. Vet Parasitol. 2007;143:147–54. DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleTable of Contents – Volume 14, Number 12—December 2008
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Christopher D. Paddock, Centers for Disease Control and Prevention, 1600 Clifton Rd NE, Mailstop G32, Atlanta, GA 30333, USA
Top