Volume 15, Number 8—August 2009
Research
Increase in Pneumococcus Macrolide Resistance, United States
Cite This Article
Citation for Media
Abstract
During year 6 (2005–2006) of the Prospective Resistant Organism Tracking and Epidemiology for the Ketolide Telithromycin surveillance study, 6,747 Streptococcus pneumoniae isolates were collected at 119 centers. The susceptibility of these isolates to macrolides was compared with data from previous years. Macrolide resistance increased significantly in year 6 (35.3%) from the stable rate of ≈30% for the previous 3 years (p<0.0001). Macrolide resistance increased in all regions of the United States and for all patient age groups. Rates were highest in the south and for children 0–2 years of age. Lower-level efflux [mef(A)]–mediated macrolide resistance decreased in prevalence to ≈50%, and highly resistant [erm(B) + mef(A)] strains increased to 25%. Telithromycin and levofloxacin susceptibility rates were >99% and >98%, respectively, irrespective of genotype. Pneumococcal macrolide resistance in the United States showed its first significant increase since 2000. High-level macrolide resistance is also increasing.
Antimicrobial drug treatment of community-acquired respiratory tract infections (RTIs) is usually initiated when the causative pathogen has not been documented. Treatment is therefore chosen empirically on the basis of potential pathogens and their antimicrobial susceptibility. Streptococcus pneumoniae is the major pathogen responsible for community-acquired RTIs (1), and treatment guidelines advise the use of agents that provide adequate coverage of this pathogen (2).
Although macrolides such as azithromycin and clarithromycin are active against S. pneumoniae and are in widespread clinical use, increasing in vitro bacterial resistance may have compromised their use. Resistance to macrolides in S. pneumoniae increased steadily during the 1990s; however, recent surveillance studies indicate that resistance may have plateaued at ≈30% in the United States (3–5). Although the link between in vitro resistance and clinical outcome is not fully understood, recent studies provide evidence that infection with macrolide-resistant pneumococci is a notable risk factor for failure of macrolide therapy in community-acquired RTIs (6–9).
Resistance to macrolides in S. pneumoniae is mediated by 2 major mechanisms: target modification caused by a ribosomal methylase encoded by the erm(B) gene or drug efflux encoded by the mef(A) gene. High-level macrolide resistance (MIC required to inhibit growth in 90% of organisms [MIC90] >32 μg/mL) is usually associated with erm(B), whereas mef(A)-mediated resistance, the most prevalent mechanism in the United States (10), usually results in lower-level resistance (MIC90 1–4 μg/mL) (11,12). Results from the Prospective Resistant Organism Tracking and Epidemiology for the Ketolide Telithromycin (PROTEKT US) surveillance study, covering isolates collected during 2000–2004, indicate that the prevalence of mef(A) is decreasing, and isolates harboring erm(B) and mef(A) genes are becoming increasingly common (13). In addition, isolates carrying only the mef(A) gene showed a higher-level resistance (MIC90 = 16 μg/mL) than observed previously (10). This analysis reports results from year 6 of PROTEKT US (2005–2006), focusing on macrolide-resistance rates and mechanisms in S. pneumoniae isolates collected from patients with community-acquired RTIs.
To reduce bias when interpreting trends, we restricted the analysis to S. pneumoniae isolates collected from the 119 centers that had previously provided isolates for year 5 of the study. Isolates were collected from patients in whom clinical acute/chronic bacterial sinusitis, acute/chronic otitis media, acute bacterial exacerbations of chronic bronchitis, chronic obstructive pulmonary disease, or community-acquired pneumonia had been diagnosed. Specimen sources included ear, blood, bronchoalveolar lavage, sinus aspirate, and sputum. Isolates were included from adults and children.
MICs for the antimicrobial agents were determined at the Central Microbiology Institute (CMI; Portland, OR, USA) by using the Clinical and Laboratory Standards Institute (CLSI) broth microdilution method (14) and were interpreted by using CLSI breakpoints (15). The breakpoints used for amoxicillin-clavulanate were <2 μg/mL (susceptible), 4 μg/mL (intermediate), and >8 μg/mL (resistant). Breakpoints used for cefpodoxime were <0.5 μg/mL (susceptible), 1 μg/mL (intermediate), and >2 μg/mL (resistant). Erythromycin-resistant (MIC >1 μg/mL) isolates were analyzed for the presence of erm(B), mef(A), and erm(TR) macrolide resistance genes by using a multiplex TaqMan PCR assay (16).
Of 6,747 S. pneumoniae isolates collected at 119 centers in year 6 of PROTEKT US, 2,381 (35.3%) showed in vitro resistance to erythromycin; this result compares with 1,907/6,257 (30.5%) in year 5. Resistance rates for azithromycin and clarithromycin in year 6 were 35.3% and 35.2%, respectively. Erythromycin resistance was stable at ≈30% in years 3, 4, and 5. Analysis of isolates from centers common to years 3–6 showed a significantly higher rate for year 6 than for years 3–5 (p<0.0001 by χ2 test).
Erythromycin resistance varied considerably by geography; the highest rates were in the North Central, Southeast, and South Central regions (Table 1). However, the rate of resistance increased from year 5 and year 6 in all 6 regions (Table 1).
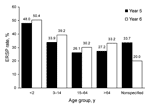
Erythromycin resistance increased from year 5 to year 6 in all patient age groups; the highest rates of resistance occurred in isolates collected from children 0–2 years of age (year 5: 423/882 [48.0%]; year 6: 533/1,058 [50.4%]) (Figure 1).
Erythromycin resistance was less frequent in isolates collected from blood than in those collected from other sources (487/1,801 [27.0%] vs. 1,894/4,946 [38.3%]).The proportion of erythromycin-resistant S. pneumoniae (ERSP) isolates exhibiting high-level resistance to erythromycin (MIC >32 μg/mL) was 18.0% in year 6 compared with 13.4% in year 5.
Coresistance to penicillin (oral penicillin V for nonmeningitis isolates) was exhibited by 14.8% of ERSP isolates collected in year 6 compared with 13.2% of ERSP isolates collected at the same centers in year 5. Among all S. pneumoniae isolates, resistance to amoxicillin-clavulanate increased from 5.2% in year 5 to 8.1% in year 6; resistance to the third-generation oral cephalosporin, cefpodoxime, increased less substantially (19.4% in year 5 vs. 20.5% in year 6).
Genotyping
The distribution of genotypes among ERSP isolates changed from year 5 to year 6. Lower-level efflux mef(A)-mediated macrolide resistance decreased, while high-level erm(B) with or without mef(A) increased (Table 2). In year 6, just over half of ERSP isolates showed mef(A) resistance; nearly one quarter were positive for erm(B) and mef(A). Analysis of isolates from centers common to years 3–6 of the study indicated a significant decreasing prevalence of mef(A) and significantly increasing prevalence of erm(B) ± mef(A) (p<0.0001 and p = 0.0033, respectively, by χ2 test).
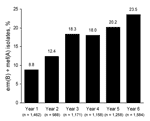
ERSP isolates from patients 0–2 years of age showed the highest frequency of the erm(B) + mef(A) genotype (38.6% in year 6 compared with 35.5% in year 5). With the exception of years 3–4, the proportion of isolates harboring erm(B) and mef(A) throughout the 6 years of the PROTEKT US study has trended upward (Figure 2).
Most (398/575 [69.2%]) of the erm(B) + mef(A) isolates from year 6 were serotype 19A; most of the remainder (154/575 [26.8%]) were serotype 19F. Overall, 72.8% of year 6 ERSP isolates were susceptible to amoxicillin-clavulanate. However, amoxicillin-clavulanate susceptibility varied considerably between genotypes; <10% of isolates carrying erm(B) and mef(A) genes were susceptible to this agent compared with >90% of isolates harboring either gene alone (Table 3). MIC50 and MIC90 for amoxicillin-clavulanate among erm(B) + mef(A) ERSP isolates were both >8 μg/mL; these were also the values for 19A and 19F strains. By contrast, >98% of ERSP isolates were susceptible to levofloxacin and >99% were susceptible to telithromycin. The genotypic mechanism of erythromycin resistance had little impact on susceptibility to either of these agents (Table 3).
These findings from PROTEKT US indicate that pneumococcal macrolide resistance has demonstrated its first significant increase since the study began in 2000 (4,13,17). Whether the increase from ≈30% to 35% represents the start of a new upward trend will become evident only when results of surveillance studies in future years become available. However, it is worth noting that another smaller US surveillance study recently reported an azithromycin resistance rate of 34% in S. pneumoniae isolates collected during the same 2005–2006 respiratory infection season as this analysis (18). A further sustained rise in macrolide resistance would be a major cause for concern because macrolides, such as azithromycin and clarithromycin, remain in widespread use for the treatment of community-acquired RTIs in the United States.
The increase in ERSP isolates from year 5 to year 6 in all 6 regions of the country indicates a lack of specific local factors that might explain this sudden increase. Even so, resistance continued to be higher in some regions (Southeast, North Central, and South Central) than in others. Higher rates of macrolide resistance in the southern states aligns with a recent retrospective cohort study involving 1,574 patients with pneumococcal bacteremia, which identified residence in the southern United States as a risk factor for infection with macrolide-nonsusceptible pneumococci (9).
Other potential explanations for the increase in macrolide resistance include increased use and/or inappropriate prescription of macrolides. Pneumococcal macrolide resistance in S. pneumoniae has been linked in several studies with increased consumption of macrolides in general and of azithromycin in particular (19). Other factors associated with pneumococcal macrolide resistance are recent use of antimicrobial drugs, age extremes, and daycare attendance (6,8). However, which (if any) of these factors might explain the trends reported here are not clear.
Another concern arising from this report is the continuing change in the distribution of macrolide-resistance genotypes. Although mef(A), usually associated with lower-level macrolide resistance, remains the most prevalent genotype, it now accounts for only about half of all ERSP isolates. Isolates carrying mef(A) continue to be replaced by strains that harbor mef(A) and erm(B) genes. Data from the first 4 years of PROTEKT US showed that the proportion of S. pneumoniae isolates positive for erm(B) and mef(A) genes increased from 9.3% to 19.1% from Fall of 2000 through spring of 2001 and the same for subsequent years through spring of 2004 while isolates positive for the mef(A) gene decreased over this time from 69.0% to 60.7% (p = 0.03) (10). A Canadian study found a significant increase of 8% (from 4% to 12%) in the prevalence of dual erm(B) and mef(A)-positive S. pneumoniae isolates (p<0.05) between Fall and spring seasons of each year (1998–2004); this increase coincided with a 17% decrease in high-level erm(B)-mediated resistance and a 5% increase in the proportion of isolates carrying only the mef(A) gene (20). The latest PROTEKT US data show that these trends are continuing; nearly one quarter of year 6 ERSP isolates have both resistance genes, and the frequency of this genotype is approaching 40% in isolates from children.
The increased prevalence of isolates harboring erm(B) and mef(A) genes is most likely due to the recent expansion in the US and elsewhere of a multidrug-resistant serotype 19A pneumococcal clone that carries both resistance genes (21,22). The expansion of this clonal variant resistance provides at least a partial explanation for the greater frequency of high-level erythromycin resistance observed in year 6 compared with that of the previous year. Most erm(B) + mef(A) strains show high-level resistance to macrolides (MIC >32 μg/mL). In addition, although mef(A) is traditionally associated with lower-level macrolide resistance (MIC 1–4 μg/mL), recent data suggest that the macrolide MICs for a growing proportion of mef(A) isolates exceed 16 μg/mL (10).
Because most erm(B) + mef(A) strains show multidrug resistance (21), their increased prevalence may compromise the effectiveness of other commonly used antimicrobial therapies. For example, although >90% of all S. pneumoniae isolates collected in year 6 of PROTEKT US exhibited in vitro susceptibility to amoxicillin-clavulanate, <10% of erm(B) + mef(A) isolates tested in this analysis were susceptible to this agent. Moreover, the MIC distribution for amoxicillin-clavulanate within the erm(B) + mef(A) isolates (MIC50 and MIC90 both >8 μg/mL) suggests that no oral β-lactam antimicrobial drug may be available that can provide adequate concentrations to eradicate these increasingly prevalent strains. On the other hand, the fluoroquinolone levofloxacin and the ketolide telithromycin continue to show good activity against ERSP isolates, with little impact of resistance genotype on their respective activities.
Our study is subject to several potential limitations. A major potential limitation inherent in surveillance studies that measure in vitro antimicrobial drug resistance is their clinical relevance. Although an association between in vitro resistance and adverse clinical outcome remains generally unproven for most respiratory infections, an increasing number of studies indicate that infection with macrolide-resistant pneumococci is associated with clinical failure (6–9,22). Furthermore, clinical failures have been associated with mef(A)- and erm(B)-mediated resistance (6,23,24). A second potential limitation of this study is the derivation of resistance rates from collection centers where a predetermined number of isolates were to be collected and may not entirely reflect those found more widely.
These data from PROTEKT US year 6 indicate that in vitro pneumococcal macrolide resistance may not have plateaued as previously thought. Continued surveillance of erythromycin resistance in general, and of highly resistant erm(B) + mef(A) strains in particular, is warranted.
Dr Jenkins is a professor of pathology and laboratory medicine at the Weill Cornell College of Medicine and director of clinical microbiology at New York Presbyterian Hospital/Weil Cornell Medical Center. His research focuses on mechanisms of antibacterial resistance.
Dr Farrell is an associate professor in the Department of Laboratory Medicine and Pathobiology, Faculty of Medicine, University of Toronto, and a clinical microbiologist and head of molecular diagnostics at the Ontario Agency for Health Protection and Promotion, Public Health Laboratory, Toronto. His research focuses on mechanisms and molecular epidemiology of antimicrobial resistance.
Acknowledgments
We thank colleagues throughout the United States for the supply of bacterial isolates as part of the PROTEKT US study and the Quotient Bioresearch Ltd and CMI PROTEKT teams who performed the MIC determinations and genotyping.
The PROTEKT US study is supported by Sanofi-Aventis US, Inc. Editorial support for this manuscript was also provided by Sanofi-Aventis US, Inc.
References
- File TM. The epidemiology of respiratory tract infections. Semin Respir Infect. 2000;15:184–94. DOIPubMedGoogle Scholar
- Mandell LA, Wunderink RG, Anzuetto A, Bartlett JG, Campbell GD, Dean NC, Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44:S27–72. DOIPubMedGoogle Scholar
- Doern GV, Richter SS, Miller A, Miller N, Rice C, Heilmann K, Antimicrobial resistance among Streptococcus pneumoniae in the United States: have we begun to turn the corner on resistance to certain antimicrobial classes? Clin Infect Dis. 2005;41:139–48. DOIPubMedGoogle Scholar
- Jenkins SG, Brown SD, Farrell DJ. Trends in antibacterial resistance among Streptococcus pneumoniae isolated in the USA: update from PROTEKT US years 1–4. Ann Clin Microbiol Antimicrob. 2008;7:1. DOIPubMedGoogle Scholar
- Sahm DF, Benninger MS, Evangelista AT, Yee YC, Thornsberry C, Brown NP. Antimicrobial resistance trends among sinus isolates of Streptococcus pneumoniae in the United States (2001–2005). Otolaryngol Head Neck Surg. 2007;136:385–9. DOIPubMedGoogle Scholar
- Daneman N, McGeer A, Green K, Low DE. Macrolide resistance in bacteremic pneumococcal disease: implications for patient management. Clin Infect Dis. 2006;43:432–8. DOIPubMedGoogle Scholar
- File TM Jr. Clinical implications and treatment of multiresistant Streptococcus pneumoniae pneumonia. Clin Microbiol Infect. 2006;12(Suppl. 3):31–41. DOIPubMedGoogle Scholar
- Klugman KP. Clinical impact of antibiotic resistance in respiratory tract infections. Int J Antimicrob Agents. 2007;29(Suppl. 1):S6–10. DOIPubMedGoogle Scholar
- Neuman MI, Kelley M, Harper MB, File TM Jr, Camargo CA Jr. Factors associated with antimicrobial resistance and mortality in pneumococcal bacteremia. J Emerg Med. 2007;32:349–57. DOIPubMedGoogle Scholar
- Farrell DJ, File TM, Jenkins SG. Prevalence and antibacterial susceptibility of mef(A)-positive macrolide-resistant Streptococcus pneumoniae over 4 years (2000 to 2004) of the PROTEKT US Study. J Clin Microbiol. 2007;45:290–3. DOIPubMedGoogle Scholar
- Hoban DJ, Doern GV, Fluit AC, Roussel-Delvallez M, Jones RN. Worldwide prevalence of antimicrobial resistance in Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis in the SENTRY Antimicrobial Surveillance Program, 1997–1999. Clin Infect Dis. 2001;32(Suppl. 2):S81–93. DOIPubMedGoogle Scholar
- Shortridge VD, Doern GV, Brueggemann AB, Beyer JM, Flamm RK. Prevalence of macrolide resistance mechanisms in Streptococcus pneumoniae isolates from a multicenter antibiotic resistance surveillance study conducted in the United States in 1994–1995. Clin Infect Dis. 1999;29:1186–8. DOIPubMedGoogle Scholar
- Jenkins SG, Farrell DJ, Patel M, Lavin BS. Trends in anti-bacterial resistance among Streptococcus pneumoniae isolated in the USA, 2000–2003: PROTEKT US years 1–3. J Infect. 2005;51:355–63. DOIPubMedGoogle Scholar
- Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard. Document M7–A7 ed. Wayne (PA): The Institute; 2006.
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: eighteenth informational supplement. Document M100–S18 ed. Wayne (PA): The Institute; 2008.
- Shackcloth J, Williams L, Farrell DJ. Streptococcus pneumoniae and Streptococcus pyogenes isolated from a paediatric population in Great Britain and Ireland: the in vitro activity of telithromycin versus comparators. J Infect. 2004;48:229–35. DOIPubMedGoogle Scholar
- Felmingham D, Reinert RR, Hirakata Y, Rodloff A. Increasing prevalence of antimicrobial resistance among isolates of Streptococcus pneumoniae from the PROTEKT surveillance study, and comparative in vitro activity of the ketolide, telithromycin. J Antimicrob Chemother. 2002;50(Suppl. S1):25–37.
- Critchley IA, Brown SD, Traczewski MM, Tillotson GS, Janjic N. National and regional assessment of antimicrobial resistance among community-acquired respiratory tract pathogens identified in a 2005–2006 U.S. Faropenem surveillance study. Antimicrob Agents Chemother. 2007;51:4382–9. DOIPubMedGoogle Scholar
- Bergman M, Huikko S, Huovinen P, Paakkari P, Seppala H. Macrolide and azithromycin use are linked to increased macrolide resistance in Streptococcus pneumoniae. Antimicrob Agents Chemother. 2006;50:3646–50. DOIPubMedGoogle Scholar
- Wierzbowski AK, Nichol K, Laing N, Hisanaga T, Nikulin A, Karlowsky JA, Macrolide resistance mechanisms among Streptococcus pneumoniae isolated over 6 years of Canadian Respiratory Organism Susceptibility Study (CROSS) (1998–2004). J Antimicrob Chemother. 2007;60:733–40. DOIPubMedGoogle Scholar
- Farrell DJ, Jenkins SG, Brown SD, Patel M, Lavin BS, Klugman KP. Emergence and spread of Streptococcus pneumoniae with erm(B) and mef(A) resistance. Emerg Infect Dis. 2005;11:851–8.PubMedGoogle Scholar
- Pichichero ME, Casey JR. Emergence of a multiresistant serotype pneumoccal strain not included in the 7-valent conjugate vaccine as an otopathogen in children. JAMA. 2007;298:1772–8. DOIPubMedGoogle Scholar
- Lonks JR, Garau J, Gomez L, Xercavins M, Odenholt-Tornqvist I, Gareen IF, Failure of macrolide antibiotic treatment in patients with bacteremia due to erythromycin-resistant Streptococcus pneumoniae. Clin Infect Dis. 2002;35:556–64. DOIPubMedGoogle Scholar
- van Kerkhoven D, Peetermans WE, Verbist L, Verhaegen J. Breakthrough pneumococcal bacteraemia in patients treated with clarithromycin or oral β-lactams. J Antimicrob Chemother. 2003;51:691–6. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 15, Number 8—August 2009
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Stephen G. Jenkins, New York-Presbyterian Hospital, Weill Cornell Medical College, 525 E 68th St, Starr 737A, New York, NY 10065, USA
Top