Volume 16, Number 2—February 2010
Letter
Hemorrhagic Fever with Renal Syndrome, Vietnam
To the Editor: Hantaviruses are primarily rodent borne and can cause hemorrhagic fever with renal syndrome (HFRS) in persons who inhale aerosolized excreta from infected rodents. The clinical characteristics of HFRS are fever, hemorrhage, and varying degrees of renal and hepatic dysfunction. Although HFRS is endemic primarily to Eurasian regions, there is serologic evidence of hantavirus infections in rodents and humans worldwide (1). Little is known about the occurrence of hantavirus infection in rodents or humans in Vietnam. One study found 5.4% prevalence of antibodies against Hantaan 76–118 and Puumala strains among residents of the Hanoi Metropolitan (2), whereas another study in southern Vietnam did not find evidence of hantavirus infection in humans (3). We describe autochthonous HFRS from Vietnam, possible reservoir hosts, and the follow-up investigation, which implies the presence of a strain of Seoul virus (SEOV).
The case-patient was a previously healthy 25-year-old nurse working in a referral hospital and residing in a semiurban district of Ho Chi Minh City. On September 23, 2008, she was admitted to the referral hospital with a history of high fever, chills, myalgia, nausea, vomiting, hematuria, and abdominal and lower back pain for 3 days. Physical examination showed a body temperature of >39°C, petechiae, mild dehydration and hypotension, with otherwise unremarkable vital signs. Hematologic tests showed 13,300 leukocytes/mm3, 167,000 thrombocytes/mm3, and hematocrit of 31%. Urinalysis showed grave hematuria (3+), proteinuria (2+), and leukouria (2+).
Three days after admission, acute renal failure with relative oliguria (0.85 L/24h) developed, as well as uremia (26.4 mg/dL), creatinemia (0.98 mg/dL), and abnormal liver function (aspartate aminotransferase 49 U/L and alanine transferase 60 U/L). The following day the patient had dyspnea and became agitated. Ultrasound examination showed pleural effusion, parietal pericardial effusion, peritoneal ascites, hepatomegaly, and renal thickness. Six days after admission, diuretic problems developed in the patient (3.7 L/24 h), her dyspnea resolved, and she became afebrile. Ten days after admission, the patient’s hematuria resolved, and renal and liver functions gradually recovered; she was discharged after 29 days of hospitalization.
Immunoglobulin (Ig) M and IgG against Hantaan recombinant nucleocapsid protein antigen were detected in the case-patient’s acute-phase and convalescent-phase serum samples, respectively, by ELISA (4,5). The presence of antihantavirus IgG was confirmed by immunofluorescent antibody (IFA) assay using whole hantavirus antigen and Western blot using hantavirus CL-1 strain (6,7). In further analysis, neutralization antibodies against SEOV strain SR-11 were detected by focus reduction neutralization test (8). The viral RNA, however, was not detectable in the acute-phase blood sample by reverse transcription–PCR (RT-PCR) (9). Other serologic tests were performed for dengue fever, typhoid fever, hepatitis B, and malaria; results of culture of blood and urine for bacteria were negative.
Following confirmation of the diagnosis, close contacts of the patient were investigated. Two family members of the patient did not have any symptoms compatible with HFRS; their serum samples were tested and found negative for antihantavirus IgG. Because the patient was a nurse, possible nosocomial transmission and sources were also investigated and excluded. On the basis of the patient’s strong history of exposure to rodents at home, further investigation focused on the domestic rodent population.
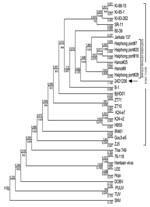
From October 14 through 16, 2008, 110 rodent traps were set within and surrounding the patient’s house. The total catch was 32 rodents, of which 16 were Rattus norvegicus, 7 R. exulans, 5 R. argentiventer, and 4 Bandicota indica. By using ELISA, IFA, and Western blot, antihantavirus IgG was detected in serum from 7 rats, of which 5 were R. norvegicus, 1 R. argentiventer, and 1 B. indica. Further analysis using RT-PCR identified 2 SEOV strains from R. norvegicus and R. argentiventer captured in the patient’s house. The M segment of 1 identified SEOV strain (24D1208) was sequenced and compared with 22 SEOV strains, 6 of which were from R. norvegicus rats captured in urban areas of North Vietnam. Phylogenetic analysis showed that this SEOV belonged to the Vietnamese SEOV genotype (Figure).
We describe a clinical case of hantavirus infection and its potential rodent reservoir occurring in Vietnam. The clinical manifestations of the case-patient were compatible with SEOV infection, which is responsible for a moderate form of HFRS (10). Also, HFRS caused by SEOV occurs in urban rather than rural areas, unlike other hantavirus infections. Our epidemiologic findings were compatible with other studies indicating the source of infection was the case-patient’s home, the only place where she had a history of exposure to rodents. Although viral RNA could not be obtained from the case-patient for genotyping, the genomic comparison of the viral strains from rodents captured in the case-patient’s home and elsewhere in Vietnam suggested that the source of infection was local rodents. This report provides additional evidence that hantavirus infection is a worldwide problem and is likely underdiagnosed in Vietnam and other countries where simple standardized laboratory diagnostics are not widely available.
Acknowledgments
We thank Phan Ngoc Nam, Ha Van Loi, and Tran Luong Anh for their assistance in the case investigation. We also thank Rika Endo for her assistance with the laboratory work and Nguyen Dac Tho and his team for their assistance in the rodent investigation.
This work was supported by a cooperative grant from the Pasteur Institute of Ho Chi Minh City, Vietnam, and the Hokkaido University Graduate School of Medicine, Sapporo, Japan.
References
- Bi Z, Formenty PBH, Roth CE. Hantavirus infection: a review and global update. J Infect Dev Ctries. 2008;2:3–23. DOIPubMedGoogle Scholar
- Rollin PE, Nawrocka E, Rodhain F. Serological data on hemorrhagic fever with renal syndrome in Southeast Asia. Bull Soc Pathol Exot Filiales. 1986;79:473–5.PubMedGoogle Scholar
- Lee PW, Svedmyr A, Gajdusek DC, Gibbs CJ, Nystrom K. Antigenic difference between European and East Asian viruses causing haemorrhagic fever with renal syndrome. Lancet. 1981;2:256–7.PubMedGoogle Scholar
- Araki K, Yoshimatsu K, Ogino M, Ebihara H, Lundkvist A, Kariwa H, Truncated hantavirus nucleocapsid proteins for serotyping Hantaan, Seoul, and Dobrava hantavirus infections. J Clin Microbiol. 2001;39:2397–404. DOIPubMedGoogle Scholar
- Miyamoto H, Kariwa H, Araki K, Lokugamage K, Hayasaka D, Cui BZ, Serological analysis of hemorrhagic fever with renal syndrome (HFRS) patients in Far Eastern Russia and identification of the causative hantavirus genotype. Arch Virol. 2003;148:1543–56. DOIPubMedGoogle Scholar
- Schmidt J, Jandrig B, Klempa B, Yoshimatsu K, Arikawa J, Meisel H, Nucleocapsid protein of cell culture-adapted Seoul virus strain 80–39: analysis of its encoding sequence, expression in yeast and immunoreactivity. Virus Genes. 2005;30:37–48. DOIPubMedGoogle Scholar
- Yoshimatsu K, Arikawa J, Kariwa H. Application of a recombinant baculovirus expressing hantavirus nucleocapsid protein as a diagnostic antigen in IFA test: cross reactivities among 3 serotypes of hantavirus which causes hemorrhagic fever with renal syndrome (HFRS). J Vet Med Sci. 1993;55:1047–50.PubMedGoogle Scholar
- Ogino M, Ebihara H, Lee BH, Araki K, Lundkvist A, Kawaoka Y, Use of vesicular stomatitis virus pseudotypes bearing Hantaan or Seoul virus envelope proteins in a rapid and safe neutralization test. Clin Diagn Lab Immunol. 2003;10:154–60.PubMedGoogle Scholar
- Zuo SQ, Zhang PH, Jiang JF, Zhan L, Wu XM, Zhao WJ, Seoul virus in patients and rodents from Beijing, China. Am J Trop Med Hyg. 2008;78:833–7.PubMedGoogle Scholar
- Lee HW. Hemorrhagic fever with renal syndrome in Korea. Rev Infect Dis. 1989;11(Suppl 4):S864–76.PubMedGoogle Scholar
Figure
Cite This ArticleRelated Links
Table of Contents – Volume 16, Number 2—February 2010
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Tran Minh Nhu Nguyen, Vietnam Field Epidemiology Training Program Office, 63 Hoang Cau St, Hanoi, Vietnam
Top