Volume 16, Number 3—March 2010
Dispatch
Candidatus Bartonella mayotimonensis and Endocarditis
Cite This Article
Citation for Media
Abstract
We describe a new Bartonella species for which we propose the name Candidatus Bartonella mayotimonensis. It was isolated from native aortic valve tissue of a person with infective endocarditis. The new species was identified by using PCR amplification and sequencing of 5 genes (16S rRNA gene, ftsZ, rpoB, gltA, and internal transcribed spacer region).
Bartonella species are small, fastidious, gram-negative, intracellular bacteria that cause culture-negative infective endocarditis. Six species have been documented to cause endocarditis in humans: B. quintana (1), B. henselae (2), B. elizabethae (3), B. vinsonii subsp. berkhoffii (4), B. koehlerae (5), and B. alsatica (6). We report a case of culture-negative endocarditis caused by a new Bartonella species, for which we propose the name Candidatus Bartonella mayotimonensis.
A 59-year-old man was initially hospitalized at Sartori Memorial Hospital (Cedar Falls, IA, USA) from April 14 through 19, 2008, for progressive shortness of breath, weight loss, fatigue, and altered mental status. He was then transferred to the Mayo Clinic (Rochester, MN, USA). Physical examination identified a new diastolic heart murmur. He was afebrile and did not have peripheral stigmata of endocarditis. Two sets of blood cultures obtained before antimicrobial drug therapy showed negative results for all bacteria tested after 5 days of incubation. A transesophageal echocardiogram showed a bicuspid aortic valve, mobile components on the left cusp of the aortic valve suggesting vegetations, and a 5.3-cm ascending aortic aneurysm. Empiric antimicrobial drug therapy, including vancomycin and ceftriaxone, was initiated. Subsequently, acute renal dysfunction, possibly secondary to vancomycin exposure, developed in the patient.
The patient lived alone on a farm in Iowa, USA, and had not had recent exposure to animals. However, he had observed murine fecal droppings in his house and mice on the farm. He had had a house cat for 18 years until its death a few years before his hospitalization and had intermittent contact with cats when he visited his daughter.
Serum immunoglobulin G titers were positive for B. henselae and B. quintana (>1,024). Oral doxycycline and rifampin were prescribed for treatment of presumed Bartonella endocarditis. Gentamicin was not administered because of development of the acute renal dysfunction. Two weeks later, he underwent aortic valve and aortic root replacement. Results of gram staining, acid-fast staining, fungal staining, anaerobic bacterial culture, aerobic bacterial culture, mycobacterial culture, and fungal culture on resected aortic valve tissue were negative for Bartonella species.
PCR performed at the Mayo Clinic on resected aortic valve tissue detected part of the citrate synthase gene (gltA) of Bartonella species. However, the melting temperature was not characteristic of B. quintana or B. henselae (7). Oral doxycycline and rifampin were continued for 12 weeks after aortic valve resection. The patient was well and had no signs of relapsing infection at a follow-up visit 11 months after valve surgery.
Aortic valve tissue and serum were tested at the Unité des Rickettsies, Marseille, France. B. quintana Oklahoma, B. henselae Houston (ATCC 49882), B. vinsonii subsp. berkhoffii (URBVAIE25), B. vinsonii subsp. arupensis (ATCC 700727), and B. alsatica (CIP 105477 T) strains were used for immunofluorescent assays and Western blotting (6). Valve tissue was injected into human endothelial cells in a shell vial assay and onto Columbia 5% sheep blood agar plates and incubated at 37°C in an atmosphere of 5% CO2 as described (6).
A Bartonella species was detected in a shell vial by immunofluorescence after 15 days of culture; identification was confirmed by PCR. DNA was extracted from valve specimen and injected cells by using the QIAamp Tissue Kit (QIAGEN, Hilden, Germany). DNA was used as a template in a genus Bartonella Lightcycler assay with primers and a Taqman probe specific for the internal transcribed spacer (ITS) gene (6) and in standard PCR assays specific for the 16S rRNA, ITS, rpoB, gltA, and ftsZ genes (8). Sequences from both DNA strands were determined twice for all PCR products. These products were resolved in an ABI 3100 automated sequencer (PerkinElmer, Waltham, MA, USA). Sterile water was used as a negative control in each assay. Percentages of similarity among sequences were determined by using MEGA 2.1 software (9). Phylogenetic relationships among Bartonella strains were inferred from concatenated sequences by using MEGA 2.1 software (9).
Surgically resected aortic valve tissues were fixed in formalin, embedded in paraffin, and sectioned to a thickness of 5 μm. Sections were stained with periodic acid–Schiff, Giemsa, Gram, Grocott-Gomori methenamine silver, and Warthin-Starry stains. Immunohistochemical analysis was performed by using a procedure described elsewhere (10) and polyclonal antibody against B. vinsonii at a dilution of 1:1,000.
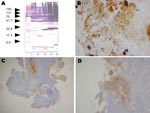
Serum samples showed immunoglobulin G endpoint titers of 50 against all Bartonella species tested by immunofluorescent assay. Western blot results were positive and characteristic of Bartonella infection (Figure 1, panel A). Results of PCR (Bartonella genus Lightcycler assay and standard PCR for cardiac valve) and cell culture were positive, and amplification products of the expected size were obtained. Among known validated species, sequences obtained shared 99.1% (1,438/1,445 bp) homology with B. tribocorum, B. henselae, and B. vinsonii for the 16S rRNA gene, 89.5% homology with B. grahamii for the ITS gene, 93.4% homology with B. vinsonii subsp. berkhoffii for rpoB, 91.7% homology with B. vinsonii subsp. berkhoffii for ftsZ, and 92.5% homology with B. vinsonii strain Baker for gltA. The phylogenetic position of Candidatus B. mayotimonensis among members of the genus Bartonella based on comparisons of concatenated sequences of the 5 genes is shown in Figure 2. Sequences of gltA, 16S rDNA, ftsZ, ITS, and rpoB were deposited in GenBank under accession nos. FJ376732–FJ376736.
Histologic analysis of resected aortic valve showed infective endocarditis with vegetation containing microorganisms that stained with Warthin-Starry and Giemsa. Warthin-Starry staining showed darkly stained bacilli consistent with Bartonella species (Figure 1, panel B). Results of staining with periodic acid–Schiff, Gram, and Grocott-Gomori methenamine silver were negative. Immunohistochemical analysis detected bacteria in valvular vegetations in a location superimposable with that detected by Warthin-Starry staining (Figure 1, panels C, D).
We isolated a new Bartonella species and propose that it be named Candidatus Bartonella mayotimonensis to recognize the contributing institutions (Mayo Clinic and Hôpital de la Timone, Marseille, France). This is the seventh Bartonella species documented to cause infective endocarditis in humans.
The reservoir of Candidatus B. mayotimonensis has yet to be determined. Different Bartonella species have been isolated from a variety of mammals, and each species is highly adapted to its reservoir host (11,12). The domestic cat is the primary mammalian reservoir for B. henselae (13). Other Bartonella species have been found in mammalian hosts, including rats (B. elizabethae), dogs and coyotes (B. vinsonii subsp. berkhoffii), cats (B. koehlerae), humans (B. bacilliformis and B. quintana), moles (B. talpae), voles (B. vinsonii subsp. vinsonii), cows (B. bovis [weissii]), deer (B. schoenbuchensis), and rabbits (B. alsatica) (3–6,12,14,15). Our patient had direct exposure to mice on his farm and also had intermittent contact with cats while visiting his daughter. Additional investigations are needed to determine the reservoir(s) and vector(s) for this novel bacterium.
The immunofluorescent assay, the current serologic method for diagnosis of Bartonella infection, does not distinguish among Bartonella species. Only Western blot analysis and cross-adsorption enable serologic identification of species. PCR and culture are critical when a Bartonella species is identified for the first time as a human pathogen. Newly encountered Bartonella strains should be considered a new species if a 327-bp gltA fragment shares <96.0% sequence similarity with those of validated species, and if an 825-bp rpoB fragment shares <95.4% sequence similarity with those of validated species as reported in the current case (8).
This case reinforces the hypothesis that any Bartonella species can cause human infection, including culture-negative endocarditis. Candidatus B. mayotimonensis should be added to the list of human pathogens that can cause culture-negative endocarditis.
Dr Lin was an internal medicine resident at the Mayo Clinic in Rochester, Minnesota, when the patient’s condition was investigated. She is currently an endocrinology fellow at Massachusetts General Hospital in Boston, Massachusetts. Her research interest is neuroendocrinology.
References
- Drancourt M, Mainardi JL, Brouqui P, Vandenesch F, Carta A, Lehnert F, Bartonella (Rochalimaea) quintana endocarditis in three homeless men. N Engl J Med. 1995;332:419–23. DOIPubMedGoogle Scholar
- Holmes AH, Greenough TC, Balady GJ, Regnery RL, Anderson BE, O’Keane JC, Bartonella henselae endocarditis in an immunocompetent adult. Clin Infect Dis. 1995;21:1004–7.PubMedGoogle Scholar
- Daly JS, Worthington MG, Brenner DJ, Moss CW, Hollis DG, Weyant RS, Rochalimaea elizabethae sp. nov. isolated from a patient with endocarditis. J Clin Microbiol. 1993;31:872–81.PubMedGoogle Scholar
- Roux V, Eykyn SJ, Wyllie S, Raoult D. Bartonella vinsonii subsp. berkhoffii as an agent of afebrile blood culture–negative endocarditis in a human. J Clin Microbiol. 2000;38:1698–700.PubMedGoogle Scholar
- Avidor B, Graidy M, Efrat G, Leibowitz C, Shapira G, Schattner A, Bartonella koehlerae, a new cat-associated agent of culture-negative human endocarditis. J Clin Microbiol. 2004;42:3462–8. DOIPubMedGoogle Scholar
- Raoult D, Roblot F, Rolain JM, Besnier JM, Loulergue J, Bastides F, First isolation of Bartonella alsatica from a valve of a patient with endocarditis. J Clin Microbiol. 2006;44:278–9. DOIPubMedGoogle Scholar
- Vikram HR, Bacani AK, DeValeria PA, Cunningham SA, Cockerill FR III. Bivalvular Bartonella henselae prosthetic valve endocarditis. J Clin Microbiol. 2007;45:4081–4. DOIPubMedGoogle Scholar
- La Scola B, Zeaiter Z, Khamis A, Raoult D. Gene-sequence-based criteria for species definition in bacteriology: the Bartonella paradigm. Trends Microbiol. 2003;11:318–21. DOIPubMedGoogle Scholar
- Kumar S, Tamura K, Jakobsen IB, Nei M. MEGA2: molecular evolutionary genetics analysis software. Bioinformatics. 2001;17:1244–5. DOIPubMedGoogle Scholar
- Lepidi H, Fournier PE, Raoult D. Quantitative analysis of valvular lesions during Bartonella endocarditis. Am J Clin Pathol. 2000;114:880–9. DOIPubMedGoogle Scholar
- La VD, Tran-Hung L, Aboudharam G, Raoult D, Drancourt M. Bartonella quintana in domestic cat. Emerg Infect Dis. 2005;11:1287–9.PubMedGoogle Scholar
- Rolain JM, Brouqui P, Koehler JE, Maguina C, Dolan MJ, Raoult D. Recommendations for treatment of human infections caused by Bartonella species. Antimicrob Agents Chemother. 2004;48:1921–33. DOIPubMedGoogle Scholar
- Eremeeva ME, Gerns HL, Lydy SL, Goo JS, Ryan ET, Mathew SS, Bacteremia, fever, and splenomegaly caused by a newly recognized Bartonella species. N Engl J Med. 2007;356:2381–7. DOIPubMedGoogle Scholar
- Breitschwerdt EB, Kordick DL. Bartonella infection in animals: carriership, reservoir potential, pathogenicity, and zoonotic potential for human infection. Clin Microbiol Rev. 2000;13:428–38. DOIPubMedGoogle Scholar
- Chomel BB, Boulouis HJ, Maruyama S, Breitschwerdt EB. Bartonella spp. in pets and effect on human health. Emerg Infect Dis. 2006;12:389–94.PubMedGoogle Scholar
Figures
Cite This ArticleTable of Contents – Volume 16, Number 3—March 2010
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Eleanor Y. Lin, Massachusetts General Hospital, Thier Bldg, Rm 1101, 55 Blossom St, Boston, MA 02114, USA
Top