Volume 17, Number 1—January 2011
Dispatch
Identification of Rickettsial Infections by Using Cutaneous Swab Specimens and PCR
Cite This Article
Citation for Media
Abstract
To determine the usefulness of noninvasive cutaneous swab specimens for detecting rickettsiae, we tested skin eschars from 6 guinea pigs and from 9 humans. Specimens from eschars in guinea pigs were positive for rickettsiae as long as lesions were present. Optimal storage temperature for specimens was 4°C for 3 days.
Rickettsiae are a group of obligate, intracellular, gram-negative bacteria. The family Rickettsiaceae includes the genera Rickettsia and Orientia (1). Rickettsiae are transmitted to humans by arthropods (2) and cause diseases characterized by fever, headache, rash, and vasculitis (3). An infection eschar is commonly found at the site of the arthropod bite because of local multiplication of the bacteria. Incidence of infection with rickettsiae is increasing worldwide (4) in certain disease-endemic foci, and seasonal, sporadic (5,6), and occasionally epidemic forms have been reported (7). Over the past 20 years, advances in molecular techniques and cell culture have facilitated identification of Rickettsiales, and new species and diseases have been described (4,8). Recently, a new Rickettsia species, 364D, was identified in patients from California (9).
Eschar biopsies are used for detection of Rickettsia spp., but this technique is invasive and painful for patients and is difficult to perform for certain areas of the body. Successful diagnosis in patients by using rapid, noninvasive, and painless techniques is beneficial. One study reported the usefulness of swabs of skin lesions in the diagnosis of 3 cases of Queensland tick typhus and 1 case of African tick bite fever (10). In addition, eschar crust samples were useful in the diagnosis of 1 case of infection with Orientia tsutsugamushi, the infectious agent of scrub typhus (11). To evaluate the potential usefulness of swabs of skin lesions for rickettsial diagnosis, we evaluated this procedure for eschars from 6 guinea pigs and 9 patients.
The animal study was conducted beginning in February 2009, and the human study was conducted beginning in June 2009. R. conorii, R. akari, R. rhipicephali, R. africae, R. parkeri, and O. tsutsugamushi were grown in L929 cell monolayers, purified, and titrated as reported (12). A suspension of each rickettsial species (200 μL containing 1 × 105 rickettsia) was injected intradermally into 8 shaved areas on the backs of 6 Hartley guinea pigs (1 species/guinea pig) by using aseptic procedures (12). A negative control guinea pig was infected with 200 μL (1 × 106 cells/mL) of an L929 cell suspension. Infection sites were inspected daily for skin lesions. Animals were handled according to the regulations of Décret No. 887–848 du 10/19/1987, Paris. The experimental protocol was reviewed and approved by the Institutional Animal Care Committee, Université de la Méditerranée, Marseille.
Infection with each rickettsial species caused an eschar at the infection site (12). Eschars were observed at day 3 postinfection. A sterile cotton swab (Copan Italia S. p. A., Brescia, Italy) was rotated against the eschar (3 circular motions) and stored at 4°C for 24 h. Swabs were then placed in 400 μL of phosphate-buffered saline, and DNA was extracted by using the QIAamp DNA Mini Kit (QIAGEN, Hilden, Germany). Lesions were swabbed daily until the animal showed clinical recovery (day 20 postinfection for those infected with R. akari, R. conorii, and R. rhipicephali and day 13 postinfection for those infected with R. africae, R. parkeri, and O. tsutsugamushi).
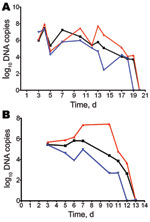
Maximum number of DNA copies for R. rhipicephali, R. akari, and R. conorii was detected on day 4 postinfection (2.27 × 107, 2.96 × 107, and 9.28 × 107copies/5 μL of swab DNA extracts, respectively (Figure 1, panel A). Maximum number of DNA copies for R. parkeri was detected on day 3 postinfection (2.66 × 105 copies/5 μL), for R. africae on day 6 postinfection (6.73 × 105 copies/5 μL), and for O. tsutsugamushi on day 10 postinfection (2.7 × 107 copies/5 μL) (Figure 1, panel B).
Effects of temperature and storage time of cotton swabs on bacterial DNA were evaluated in 3 guinea pigs infected with R. conorii. Twelve swabs per animal were obtained daily for 5 days and stored in groups of 3 at 22°C, 4°C, −20°C, or −80°C. DNA was extracted after 1, 2, or 3 days of storage. Eschars appeared by day 3 postinfection and reached their maximum size by day 7. Storage at 4°C was the optimal temperature condition for isolation of DNA (7.53 × 106 copies/5 μL vs. 1.03 × 106, 3.77 × 106, or 4.49 × 106 copies/5 μL for swab storage at 22°C, −20°C, or −80°C respectively; p = 0.0001) (Figure 2, panel A). Storage time (24 h, 48 h, and 72 h) had no effect on DNA yield (Figure 2, panel B). Temperature had a significant effect (p<0.05) on DNA yield and for the same extraction (Figure 2, panels C–E).
To demonstrate the usefulness of skin lesion swabs for detection of rickettsial infection, we used this technique with eschars from patients with suspected rickettsioses. Nine patients were included in this experiment after informed consent was obtained. This experiment was reviewed and approved by the local ethics committee (reference 09–016). DNA was extracted from swabs or skin biopsy specimens and tested by quantitative PCRs (qPCRs) (13) specific for a fragment of the citrate synthase A gene, which is conserved among spotted fever group rickettsiae, or the gene coding periplasmic serine protease of O. tsutsugamushi; β-actin gene was used as a control (14).
When rickettsial DNA was amplified in samples, specific qPCR was performed by using specific primers and probes and on the basis of clinical and epidemiologic data (Technical Appendix Table 1 ) (4). If specific rickettsial DNA was not detected, PCR amplification and sequencing were performed to identify the causative agent (4,15). R. montanensis DNA was used as a positive control, and DNA from sterile biopsy samples and sterile water were used as a negative control.
The qPCR for the β-actin gene showed cycle threshold (Ct) values of 19–23 for skin biopsy samples and 22–37 for swab samples (Table). Spotted fever group rickettsial DNA was detected in biopsy samples from 5/5 patients and swab samples from 8/9 patients (Technical Appendix Table 2). Specific qPCR showed a diagnostic result in 3/7 swabs samples and 4/5 skin biopsy samples.
We amplified R. conorii DNA from patients 1 and 2, R. africae DNA from patients 4 and 5, and R. australis DNA from patient 9. Rickettsial DNA from patients 3 and 7 showed 100% homology with the R. sibirica mongolitimonae citrate synthase A gene (GenBank accession nos. DQ097081 and DQ423370, respectively). Rickettsial DNA from patient 6 showed 99.1% homology with DNA from R. slovaca. Patient 9 was a technician who was accidentally infected by the aerosol route when handling R. australis. Only 2/11 swabs obtained from vesicular lesions of patient 9 were positive for rickettsial DNA and R. australis DNA after reamplification of primary PCR products. These samples showed 98% homology with R. australis 23S rRNA gene (GenBank accession no. AJ133711) (Technical Appendix Table 2).
Our study showed the efficacy and reliability of skin lesion swabs for molecular detection of 6 Rickettsia species (Figure 1). Rickettsial DNAs were detected by using this technique as long as eschars persisted (<19 days). For short-term storage of swabs, 4°C was the optimal temperature. Using swabs of eschars, we made a diagnosis of rickettsiosis for 8/9 patients. For patients 6, 7, and 8, for whom biopsy samples were not available, we confirmed the diagnosis by using swab samples. We also showed that for patient 9, who had a rickettsiosis but no eschar, swabbing of vesicular lesions may be useful for diagnosis, although these lesions were less sensitive than eschars.
Our results indicate that swabs of eschars can be used for molecular detection of rickettsial infections when biopsy samples are not available or biopsies are difficult to perform. We recommend that swabs be used for DNA extraction immediately after sampling or stored at 4°C until needed.
Dr Bechah is a postdoctoral fellow at the Université de la Méditerranée, Marseille, France. Her research interests are epidemic typhus, its relapsing form (Brill-Zinsser disease), and the reservoir of this disease.
Acknowledgments
We thank L. Espinosa and M. Bedotto for help with statistical analysis and design of primers and probe for R. australis detection.
This study was supported by the Hospital Clinical Research Program from the French Health Ministry (Recherche de Protéines Candidates à la Mise au Point d’un Vaccin par Étude de la Transcription de Rickettsia conorii chez l’Homme au Cours de la Fièvre Boutonneuse Méditerranéenne) (2004).
References
- Bechah Y, Capo C, Mege JL, Raoult D. Rickettsial diseases: from Rickettsia-arthropod relationships to pathophysiology and animal models. Future Microbiol. 2008;3:223–36. DOIPubMedGoogle Scholar
- Raoult D, Roux V. Rickettsioses as paradigms of new or emerging infectious diseases. Clin Microbiol Rev. 1997;10:694–719.PubMedGoogle Scholar
- Walker DH, Valbuena GA, Olano JP. Pathogenic mechanisms of diseases caused by Rickettsia. Ann N Y Acad Sci. 2003;990:1–11. DOIPubMedGoogle Scholar
- Parola P, Paddock CD, Raoult D. Tick borne rickettsioses around the world: emerging diseases challenging old concepts. Clin Microbiol Rev. 2005;18:719–56. DOIPubMedGoogle Scholar
- Paddock CD, Holman RC, Krebs JW, Childs JE. Assessing the magnitude of fatal Rocky Mountain spotted fever in the United States: comparison of two national data sources. Am J Trop Med Hyg. 2002;67:349–54.PubMedGoogle Scholar
- Hidalgo M, Orejuela L, Fuya P, Carrillo P, Hernandez J, Parra E, Rocky Mountain spotted fever, Colombia. Emerg Infect Dis. 2007;13:1058–60.PubMedGoogle Scholar
- Masters EJ, Olson GS, Weiner SJ, Paddock CD. Rocky Mountain spotted fever: a clinician’s dilemma. Arch Intern Med. 2003;163:769–74. DOIPubMedGoogle Scholar
- Raoult D. A new rickettsial disease in the United States. Clin Infect Dis. 2004;38:812–3. DOIPubMedGoogle Scholar
- Shapiro MR, Fritz CL, Tait K, Paddock CD, Nicholson WL, Abramowicz KF, Rickettsia 364D: a newly recognized cause of eschar-associated illness in California. Clin Infect Dis. 2010;50:541–8. DOIPubMedGoogle Scholar
- Wang JM, Hudson BJ, Watts MR, Karagiannis T, Fisher NJ, Anderson C, Diagnosis of Queensland tick typhus and African tick bite fever by PCR of lesion swabs. Emerg Infect Dis. 2009;15:963–5. DOIPubMedGoogle Scholar
- Lee SH, Kim DM, Cho YS, Yoon SH, Shim SK. Usefulness of eschar PCR for diagnosis of scrub typhus. J Clin Microbiol. 2006;44:1169–71. DOIPubMedGoogle Scholar
- La Scola B, Bechah Y, Lepidi H, Raoult D. Prediction of rickettsial skin eschars in humans using an experimental guinea pig model. Microb Pathog. 2009;47:128–33. DOIPubMedGoogle Scholar
- Bechah Y, Capo C, Grau GE, Raoult D, Mege JL. A murine model of infection with Rickettsia prowazekii: implications for pathogenesis of epidemic typhus. Microbes Infect. 2007;9:898–906. DOIPubMedGoogle Scholar
- Mediannikov O, Fenollar F, Socolovschi C, Diatta G, Sokhna C, Bassene H, Coxiella burnetii in humans and ticks in rural Senegal. PLoS Negl Trop Dis. 2010;4:e654. DOIPubMedGoogle Scholar
- Roux V, Rydkina E, Eremeeva M, Raoult D. Citrate synthase gene comparison, a new tool for phylogenetic analysis, and its application for the rickettsiae. Int J Syst Bacteriol. 1997;47:252–61. DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleTable of Contents – Volume 17, Number 1—January 2011
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Didier Raoult, Unité de recherche sur les maladies infectieuses et tropicales émergentes (URMITE), CNRS UMR 6236, IRD 3R198, IFR 48, Université de la Méditerranée, Faculté de Médecine, 27 Boulevard Jean Moulin, 13385 Marseille CEDEX 05, France
Top