Volume 17, Number 1—January 2011
Research
Molecular Typing of Protease-Resistant Prion Protein in Transmissible Spongiform Encephalopathies of Small Ruminants, France, 2002–2009
Figure 5
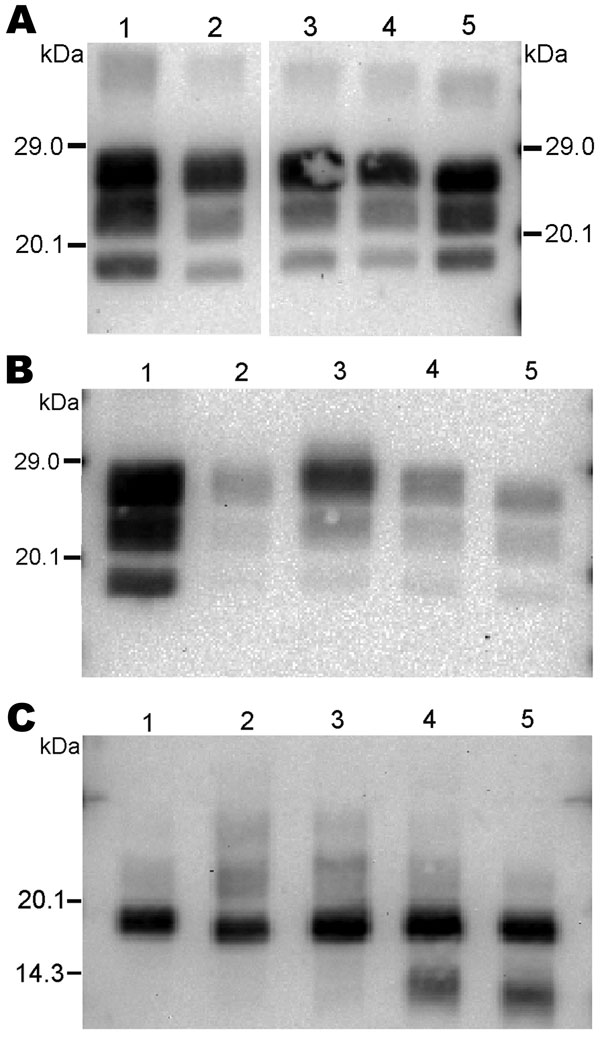
Figure 5. Western blot analysis of protease-resistant prion protein in 2 goat isolates (CH636, lane 3; 08-357, lane 4) detected by Bar233 (A), P4 (B), and SAF84 (C) antibodies. These samples were compared with an isolate from a goat naturally infected with scrapie (lane 1); an isolate from a goat experimentally infected with classical BSE (CH41x76, lane 2); and a sheep-passaged scrapie isolate (CH1641, lane 5). Samples in panel C were deglycosylated with peptide N-glycosidase F before Western blot analysis.
Page created: July 08, 2011
Page updated: July 08, 2011
Page reviewed: July 08, 2011
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.