Volume 17, Number 4—April 2011
Dispatch
High Rates of Staphylococcus aureus USA400 Infection, Northern Canada
Abstract
Surveillance of Staphylococcus aureus infections in 3 northern remote communities of Saskatchewan was undertaken. Rates of methicillin-resistant infections were extremely high (146–482/10,000 population), and most (98.2%) were caused by USA400 strains. Although USA400 prevalence has diminished in the United States, this strain is continuing to predominate throughout many northern communities in Canada.
Over the past decade, community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) infections have rapidly emerged in Canada (1). These CA-MRSA strains are causing infections in often young otherwise healthy persons with no traditional health care–associated risk factors (2), linked with increased illness severity and deaths (3), and now entering and being disseminated within health care facilities (4). In comparison to the incidence of CA-MRSA infections in large urban centers across Canada, which has been addressed through the ongoing efforts of the Canadian Nosocomial Infection Surveillance Program (1), little attention has been directed at the emerging problem of CA-MRSA or CA-methicillin-susceptible S. aureus (MSSA) in rural and northern communities of Canada. In this study, active surveillance was undertaken in 3 remote northern communities to assess the prevalence and effects of MRSA and MSSA infections.
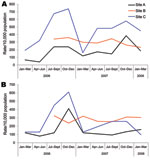
Clinically significant MRSA and MSSA isolates, identified during January 2006–March 2008, within 3 select communities (sites A–C) in northern Saskatchewan were included in this surveillance study. Site B also included 1 adjoining community, and sites A and B also included additional First Nations Reserves serviced by the community. Each site faced significant socioeconomic challenges. A total of 1,280 isolates, obtained from skin and soft tissue infections (SSTIs), urinary tract infections, upper respiratory tract infections, and lower respiratory tract infections, were identified as S. aureus. A high proportion of these isolates, 692 (54.1%) of 1,280, were MRSA. Over the 2-year study period, rates of MRSA and MSSA infections in the 3 communities ranged from 146–482/10,000 and 112–329/10,000 population, respectively. Trends of seasonality were apparent for MRSA infections, with the highest rates being observed in the third and fourth quarters of the year (Figure 1). Overall, the highest quarterly rates of MRSA and MSSA infections were observed at site C, with 738/10,000 and 610/10,000 population, respectively.
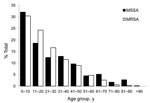
The highest proportion of MRSA (30.4%) and MSSA (32.1%) infections were identified in children <10 years of age (Figure 2). Compared to MSSA infections, MRSA infections were statistically more likely to be causing infections in persons <30 years of age (odds ratio [OR] 1.46, 95% confidence interval [CI] 1.14–1.86, p = 0.002) and less likely to be causing infections in patients >60 years of age (OR 0.33, 95% CI 0.20–0.567), p<0.001) (Figure 2). No significant difference was found in gender between those who acquired MRSA (46.7% male) and MSSA (53.3% female, 49.4% male) infections.
Most MRSA (98.6%) and MSSA (91%) isolates were obtained from SSTIs. Further analysis of SSTIs, comparing where on the body the infections were seen, showed significantly more MRSA infections in the axillae (OR 3.04, 95% CI 1.39–6.89, p = 0.004), buttocks (OR 2.1, 95% CI 1.27–3.49, p = 0.003), and trunk (OR 2.25, 95% CI 1.54–3.31, p = <0.001) than MSSA infections. MRSA infections were significantly less likely to be found in feet (OR 0.29, 95% CI 0.18–0.45, p<0.001), hands (OR 0.45, 95% CI 0.3–0.68, p<0.001), and face or head (OR 0.66, 95% CI 0.48–0.90, p = 0.009). Of the additional infection sites included in this study, MSSA infections were statistically more likely to be identified in lower respiratory tract infections (OR 5.6, 95% CI 1.5–24.62, p<0.05) and urinary tract infections (OR 6.76, 95% CI 2.87–16.71, p<0.001).
A subset of 665 isolates were further characterized by antimicrobial drug susceptibility testing (Table 1). In comparison to MSSA, MRSA were significantly more likely to be susceptible to clindamycin, erythromycin, fusidic acid, and gentamicin, but were more likely to be resistant to mupirocin (Table 1). In regards to the clindamycin-resistant isolates, 3 (18.8%) of the 16 MRSA isolates and 73 (93.6%) of the 78 MSSA isolates were inducible. For mupirocin-resistant isolates, all 328 of the MRSA isolates, but only 54 (70.1%) of the 77 MSSA isolates, displayed high level resistance (>128 μg/mL).
Pulsed-field gel electrophoresis (PFGE) showed that most MRSA isolates (372/379, 98.2%) were USA400. The remaining 7 MRSA isolates were identified as CMRSA10 (USA300, sequence type (ST) 8) (n = 5), CMRSA2 (USA100/800, ST5) (n = 1), and CMRSA8 (EMRSA15, ST22) (n = 1). As anticipated, PFGE revealed much greater genetic diversity among the MSSA strains circulating in these regions than in MRSA strains. Notably, however, most MSSA PFGE fingerprints (79.2%) were related to highly successful Canadian epidemic MRSA strains, a finding that was further confirmed by using spa typing (5) (Table 2).
MRSA isolates were more likely to harbor the genes encoding Panton-Valentine leukocidin than were MSSA isolates, 95.5% versus 5.2%, respectively. The PFGE and spa types of the 15 Panton-Valentine leukocidin–positive MSSA isolates were associated with the CA-MRSA epidemic strain types USA400, USA300, and USA1000 (Table 2).
Rates of MSSA and MRSA infections in these 3 northern Saskatchewan communities (112–482 cases/10,000 population) far exceed MRSA rates reported in the neighboring provinces of Manitoba (≈16/10,000 population) (6) and Alberta (10.7/10,000 population) (7), as well as benchmark hospital rates provided by the Canadian Nosocomial Infection Surveillance Program (3.43 cases/10,000 patient days) (1). The high rates of S. aureus infections in remote northern Saskatchewan communities has been attributed to a combination of risk factors, including overcrowding and poor housing conditions, inadequate hygiene, preexisting skin conditions, and previous high usage of antimicrobial drugs (8).
USA400 was by far the predominant strain type in all 3 communities, accounting for >98% of the MRSA isolates. USA400 was first reported in Manitoba as an outbreak in the southern region in the late 1990s, but has since spread to the northern regions of the province from 2000 to 2004 (9). USA400 was thereafter seen in a central eastern Saskatchewan community adjacent to the Manitoba border (2) and has since disseminated as far north as Nunavut (10) and southwestern Alaska (11).
Because MRSA and MSSA SSTIs tended to be identified more frequently from different body sites, it is appealing to speculate that CA-MRSA strains, such as USA400, might also colonize different body sites (e.g., axillae or intestines) more efficiently than other strains of S. aureus. This hypothesis coincides with a recent report in which nasal colonization was less likely in patients with CA-MRSA SSTIs than in those with hospital-acquired MRSA SSTIs (12). Intestinal carriage of S. aureus has been implicated as a risk factor for infection (13) and could be a strong contributor to environmental dissemination and transmission (14). This possibility was recently further supported by the results of a study in which the rectal carriage, but not nasal carriage, of USA300 was strongly associated with SSTIs in children (15). Further study is required to determine whether specific lineages of S. aureus are more proficient colonizers at non-nasal colonization sites, what host/bacteria genetic factors are involved, and whether this colonization plays a role in the high success of these CA-MRSA strain types.
To address the high rates of S. aureus infections in northern Saskatchewan, physician treatment algorithms and educational materials have been provided throughout many northern communities and schools in Saskatchewan. These materials are all freely available (www.narp.ca) and are intended to promote proper antimicrobial drug usage and hygiene to diminish the spread of S. aureus disease.
Dr Golding is a research scientist at the National Microbiology Laboratory, Winnipeg. His primary research interest focuses on antimicrobial drug resistance mechanisms, genomics, typing, and surveillance of S. aureus.
Acknowledgments
The following are members of the Northern Antibiotic Resistance Partnership: Michael Mulvey, George Golding (National Microbiology Laboratory, Winnipeg, Manitoba); Greg Horsman, Paul N. Levett, Ryan McDonald, Evelyn Nagle, Christine Schachtel, Christina Schwickrath, Arlene Obarianyk, Toni Hansen (Saskatchewan Disease Control Laboratory, Regina, Saskatchewan [SK]); Donna Stockdale, James Irvine, Brian Quinn (Population Health Unit, LaRonge, SK); Brenda Mishak-Beckman (Mamawetan Churchill River Health Region and Athabasca Health Authority, LaRonge); Jill Johnson (Mamawetan Churchill River Health Region, LaRonge); Mandiangu Nsungu, Shirley Woods (Northern Intertribal Health Authority, Prince Albert, SK); Mohammad Khan (Kelsey Trail Health Region, Melfort, SK); Pat Malmgren (Keewatin Yatthé Health Region (Buffalo Narrows, SK); Brenda Cholin (Prairie North Health Region, North Battleford, SK); Zachary Whitecap, Barb Brooke, Matilda McKay (Red Earth First Nation, SK); Ruth Bear, Georgina Quinney, Annel Bear (Shoal Lake Cree Nation, SK); Shirley Paton, Marianna Ofner-Agostini (Public Health Agency of Canada, Ottawa, Ontario); Brian Szclarzuk, Steve Silcox (Public Health Agency of Canada, Winnipeg); John Embil, Kirsten Bergstrom, Amanda Horbal, Christine Siemens, Nadia Persaud (University of Manitoba, Winnipeg).
Funding for this study was provided by the Canadian Institutes of Health Research and the Public Health Agency of Canada.
References
- Simor AE, Gilbert NL, Gravel D, Mulvey MR, Bryce E, Loeb M, Methicillin-resistant Staphylococcus aureus colonization or infection in Canada: National surveillance and changing epidemiology, 1995–2007. Infect Control Hosp Epidemiol. 2010;31:348–56. DOIPubMedGoogle Scholar
- Mulvey MR, MacDougall L, Cholin B, Horsman G, Fidyk M, Woods S, Community-associated methicillin-resistant Staphylococcus aureus, Canada. Emerg Infect Dis. 2005;11:844–50.PubMedGoogle Scholar
- Vayalumkal JV, Whittingham H, Vanderkooi O, Stewart TE, Low DE, Mulvey M, Necrotizing pneumonia and septic shock: suspecting CA-MRSA in patients presenting to Canadian emergency departments. CJEM. 2007;9:300–3.PubMedGoogle Scholar
- Seybold U, Kourbatova EV, Johnson JG, Halvosa SJ, Wang YF, King MD, Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of health care–associated blood stream infections. Clin Infect Dis. 2006;42:647–56. DOIPubMedGoogle Scholar
- Golding GR, Campbell JL, Spreitzer DJ, Veyhl J, Surynicz K, Simor A, A preliminary guideline for the assignment of methicillin-resistant Staphylococcus aureus to a Canadian pulsed-field gel electrophoresis epidemic type using spa typing. Can J Infect Dis Med Microbiol. 2008;19:273–81.PubMedGoogle Scholar
- Larcombe L, Waruk J, Schellenberg J, Ormond M. Rapid emergence of methicillin-resistant Staphylococcus aureus (MRSA) among children and adolescents in northern Manitoba, 2003–2006. Can Commun Dis Rep. 2007;33:9–14.PubMedGoogle Scholar
- Kim J, Ferrato C, Golding GR, Mulvey MR, Simmonds KA, Svenson LW, Changing epidemiology of methicillin-resistant Staphylococcus aureus in Alberta, Canada: population-based surveillance, 2005–2008. Epidemiol Infect. .DOIPubMedGoogle Scholar
- Golding GR, Levett PN, McDonald RR, Irvine J, Nsungu M, Woods S, A comparison of risk factors associated with community-associated methicillin-resistant and –susceptible Staphylococcus aureus infections in remote communities. Epidemiol Infect. 2010;138:730–7. DOIPubMedGoogle Scholar
- Wylie JL, Nowicki DL. Molecular epidemiology of community- and health care-associated methicillin-resistant Staphylococcus aureus in Manitoba, Canada. J Clin Microbiol. 2005;43:2830–6. DOIPubMedGoogle Scholar
- Dalloo A, Sobol I, Palacios C, Mulvey M, Gravel D, Panaro L. Investigation of community-associated methicillin-resistant Staphylococcus aureus in a remote northern community, Nunavut, Canada. Can Commun Dis Rep. 2008;34:1–7.PubMedGoogle Scholar
- David MZ, Rudolph KM, Hennessy TW, Boyle-Vavra S, Daum RS. Molecular epidemiology of methicillin-resistant Staphylococcus aureus, rural southwestern Alaska. Emerg Infect Dis. 2008;14:1693–9. DOIPubMedGoogle Scholar
- Yang ES, Tan J, Eells S, Rieg G, Tagudar G, Miller LG. Body site colonization in patients with community-associated methicillin-resistant Staphylococcus aureus and other types of S. aureus skin infections. Clin Microbiol Infect. 2010;16:425–31 .DOIPubMedGoogle Scholar
- Acton DS, Plat-Sinnige MJ, van Wamel W, de Groot N, van Belkum A. Intestinal carriage of Staphylococcus aureus: how does its frequency compare with that of nasal carriage and what is its clinical impact? Eur J Clin Microbiol Infect Dis. 2009;28:115–27. DOIPubMedGoogle Scholar
- Boyce JM, Havill NL, Otter JA, Adams NM. Widespread environmental contamination associated with patients with diarrhea and methicillin-resistant Staphylococcus aureus colonization of the gastrointestinal tract. Infect Control Hosp Epidemiol. 2007;28:1142–7. DOIPubMedGoogle Scholar
- Faden H, Lesse AJ, Trask J, Hill JA, Hess DJ, Dryja D, Importance of colonization site in the current epidemic of Staphylococcal skin abscesses. Pediatrics. 2010;125:18–24. Epub 2010 Feb 15. DOIPubMedGoogle Scholar
Figures
Tables
Cite This Article1Members of the Northern Antibiotic Resistance Partnership are listed at the end of this article.
Table of Contents – Volume 17, Number 4—April 2011
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Michael R. Mulvey, National Microbiology Laboratory, 1015 Arlington St, Winnipeg, MB R3E 3R2, Canada
Top