Volume 17, Number 4—April 2011
Dispatch
Highly Pathogenic Avian Influenza Virus Infection in Feral Raccoons, Japan
Figure 2
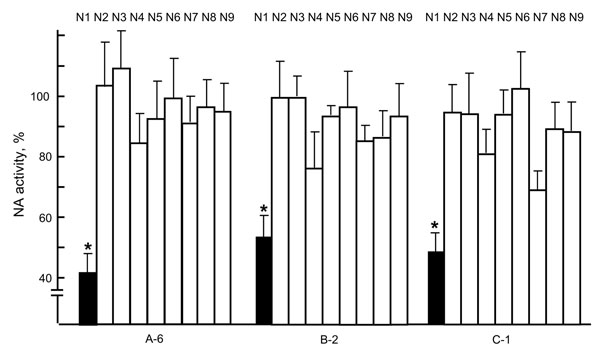
Figure 2. Neuraminidase (NA) inhibition by virus neutralization (VN)–positive raccoon serum samples. Each of the N1 to N9 viruses, consisting of A/whooper swan/Mongolia/4/05 (H5N1), A/Hong Kong/1073/99 (H9N2), A/whooper swan/Shimane/499/83 (H5N3), A/turkey/Ontario/6188/68 (H8N4), A/duck/Alberta/60/76 (H12N5), A/duck/England/56 (H11N6), A/seal/Massachusetts/1/80 (H7N7), A/duck/Ukraine/1/63 (H3N8), and A/duck/Memphis/546/74 (H11N9), was incubated with a VN-positive serum sample (A-6, B-2, or C-1) for 1 h at 37°C, and viral NA activity was then measured (6). Data are shown as percentage of activities compared with incubation with VN-negative serum samples (100%). Three independent tests were performed, and significant reduction of NA activity (p<0.05, t test with 2-tailed analysis) was observed only for N1 virus (*). HA, hemagglutinin. Error bars indicate SDs of 3 independent tests.
References
- United States Geological Survey. USGS National Wildlife Health Center. List of species affected by H5N1 (avian influenza) [cited 2010 Aug 5]. http://www.nwhc.usgs.gov/disease_information/avian_influenza/affected_species_chart.jsp
- Vandalen KK, Shriner SA, Sullivan HJ, Root JJ, Franklin AB. Monitoring exposure to avian influenza viruses in wild mammals. Mammal Rev. 2009;39:167–77. DOIGoogle Scholar
- Hall JS, Bentler KT, Landolt G, Elmore SA, Minnis RB, Campbell TA, Influenza infection in wild raccoons. Emerg Infect Dis. 2008;14:1842–8. DOIPubMedGoogle Scholar
- Roberts NM, Henzler DJ, Clark L. Serological evidence of avian influenza (H4N6) exposure in a wild-caught raccoon. Avian Dis. 2009;53:455–7. DOIPubMedGoogle Scholar
- Sullivan HJ, Blitvich BJ, VanDalen K, Bentler KT, Franklin AB, Root JJ. Evaluation of an epitope-blocking enzyme-linked immunosorbent assay for the detection of antibodies to influenza A virus in domestic and wild avian and mammalian species. J Virol Methods. 2009;161:141–6. DOIPubMedGoogle Scholar
- Itoh Y, Shinya K, Kiso M, Watanabe T, Sakoda Y, Hatta M, In vitro and in vivo characterization of new swine-origin H1N1 influenza viruses. Nature. 2009;460:1021–5.PubMedGoogle Scholar
- Root JJ, Bentler KT, Sullivan HJ, Blitvich BJ, McLean RG, Franklin AB. Antibody responses of raccoons naturally exposed to influenza A virus. [ PMID: 20370429]. Vector Borne Zoonotic Dis. 2010;10:821–3. DOIPubMedGoogle Scholar
- Kitphati R, Pooruk P, Lerdsamran H, Poosuwan S, Louisirirotchanakul S, Auewarakul P, Kinetics and longevity of antibody response to influenza A H5N1 virus infection in humans. Clin Vaccine Immunol. 2009;16:978–81. DOIPubMedGoogle Scholar
- Desrosiers R, Bloutin R, Broes A. Persistence of antibodies after natural infection with swine influenza virus and epidemiology of the infection in a herd previously considered influenza-negative. J Swine Health Prod. 2004;12:78–81.