Volume 17, Number 4—April 2011
Dispatch
Recent Clonal Origin of Cholera in Haiti
Cite This Article
Citation for Media
Abstract
Altered El Tor Vibrio cholerae O1, with classical cholera toxin B gene, was isolated from 16 patients with severe diarrhea at St. Mark’s Hospital, Arbonite, Haiti, <3 weeks after onset of the current cholera epidemic. Variable-number tandem-repeat typing of 187 isolates showed minimal diversity, consistent with a point source for the epidemic.
On October 21, 2010, isolation of toxigenic Vibrio cholerae O1 from patients with severe diarrhea was confirmed by the National Laboratory of Public Health of the Ministry of Public Health and Population in Haiti (1). These cases indicated onset of epidemic cholera in Haiti and were followed by rapid spread of the disease throughout the country. Illness occurred in the setting of major disruptions of water and sewage facilities resulting from the January 12, 2010, earthquake and associated deficiencies in local public health infrastructure (1).
Before the current epidemic, cases of cholera had not been reported in Haiti since 1960 (2), and disease had not spread into Haiti during expansion of the El Tor pandemic into Latin America that began in Peru in 1991. However, toxigenic V. cholerae O1 are present along the US Gulf Coast (3,4) and in other coastal areas in the Western Hemisphere. In conjunction with ongoing public health activities in Haiti by the University of Florida, we analyzed fecal samples from patients early in the epidemic. Data obtained on V. cholerae strain diversity and gene content improved our understanding of the epidemiology of this outbreak.
Fecal samples from 19 patients were provided by staff at St. Mark’s Hospital, Artibonite, Haiti, to University of Florida investigators on November 9, 2010, <3 weeks after onset of the epidemic. Microbial testing of samples was reviewed and approved by the University of Florida Institutional Review Board. Samples were directly plated on thiosulfate citrate bile salts sucrose agar and placed in alkaline peptone water for enrichment. Yellow colonies were identified by standard biochemical tests and confirmed by PCR for the outer membrane protein W gene (5).
V. cholerae O1 Ogawa was isolated from 16 of 19 samples. All isolates were El Tor biotype and had the El Tor type regulatory gene for phage lysogeny and the co-regulated pilus A gene identified by PCR (6,7). All isolates were newly identified altered El Tor that carried the classical cholera toxin B gene, as determined by mismatch amplification mutation assay–PCR (8).
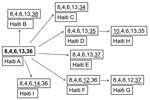
To evaluate genetic diversity, we randomly chose <20 colonies (average 14.4) from each of 13 fecal samples that were culture positive without enrichment. A total of 187 colonies were typed by using multilocus variable-number tandem-repeat (VNTR) analysis as described (9–12). We used 5 loci (VC0147, VC0437 [the VC0436-7 intergenic region], VC1650, VCA0171, and VCA0283) reported by Ghosh et al. (10) and Choi et al. (12). Only 9 sequence types (STs) were identified (Figure); all were within 1 clonal complex, and each differed from the others by 1 allele. Type A (8,4,6,13,36) (Figure) was the dominant ST, present in 9 of 13 patients (Table). In 6 of these 9 patients, A was the only type identified. As reported (10), loci on the smaller chromosome had the greatest diversity (3 alleles for VCA0171 and 5 alleles for VCA0283) compared with no variation for VC0437 or VC1650 and only 1 change in VC0147. None of these STs has been reported in studies of strains from Bangladesh, India, Vietnam, or Mozambique (9–12).
Profound disruption of sanitary and public health infrastructure in Haiti resulting from the January 12, 2010, earthquake created an environment in which rapid spread of a disease such as cholera might be expected. However, because cholera had not been reported in Haiti since 1960, its sudden appearance raises questions about its origin, which has implications for understanding transmission pathways and potential for further spread.
Estuarine and freshwater/riverine (13) environments are well-recognized reservoirs for V. cholerae and show long-term persistence of epidemic strains in the absence of human cases. The US Gulf Coast is an excellent example of this environment, and periodic cholera cases occur there, likely linked to a common clonal strain that has a characteristic pulsed-field gel electrophoresis (PFGE) banding pattern (14). Cases along the Gulf Coast have intermittently appeared since 1978 (3). Indigenously acquired cholera cases linked to seafood consumption were reported in October 2005 in Louisiana in temporal association with Hurricanes Katrina and Rita (4).
When cholera appeared in Haiti in October 2010, cases were clustered along a 20-mile stretch of the Artibonite River, and 18 (67%) of 27 hospitalized patients reported drinking untreated water from the river or canals before illness onset, which is consistent with the river as a source of infection (1). Although these data are consistent with a limited origin for the epidemic, whether these strains represent a persistent clone in the river environment or a new strain in Haiti is unknown.
Epidemic strains of V. cholerae O1 and O139 show a high degree of genetic similarity, making it difficult to separate various subgroups by using standard molecular typing approaches such as ribotyping, PFGE, and multilocus sequence typing (6). PFGE types tend to change slowly and are useful primarily for distinguishing strains in different pandemics or between continental branches of pandemics (11,14). PFGE has shown that strains from Haiti are similar to strains from southern Asia and other regions (1). Sequences from 2 isolates from Haiti were most similar to El Tor isolates from Bangladesh in 2002 and 2008 (15).
In recent studies (9–12), VNTR typing has provided greater discrimination for ongoing or established epidemic O1 and O139 serogroups. Three loci (VC0147, VC0437, and VC1650) on the large chromosome were more stable (9–11) and are likely to be considered the best loci for estimating across distances, especially because of our observation of large differences in genotypes between locations 50 miles apart in rural Bangladesh (9).
Although genotype 8,4,6 has not been seen in other studies (9–12), genotype 9,4,6 was common in Dhaka (11) and associated with O1 Ogawa, the same serotype and biotype as strains from Haiti. In the absence of a more comprehensive global VNTR database, establishing a definite source for strains from Haiti on the basis of VNTR typing is not possible. However, our findings are consistent with those of others studies implicating southern Asia as the source for these strains on the basis of deletion/insertion data for the superintegron and the sulfamethoxazole/trimethoprim resistance integron island, and from analysis of single nucleotide polymorphisms, including those in the cholera toxin gene identified in the complete sequence of the strain from Haiti (15).
Our finding of only 9 STs differing at 1 allele among 187 colonies underscores the clonality of the strains from Haiti. Given the degree of diversity in STs among environmental strains in Bangladesh, including development of almost entirely different sets of multiple ST patterns among isolates from locations 50 miles apart, lack of diversity among isolates from Haiti would support the hypothesis that the epidemic in Haiti was caused by 1 clone that had little time to undergo diversification of STs expected of strains persistent in an environmental reservoir for extended periods.
Although altered El Tor strains have evolved only within the past several decades, which also argues against a long-standing environmental source (8), VNTR analysis, with its rapid molecular clock, can be used to test theories about the origin of the strain in Haiti and provide a unique opportunity to follow diversification of this clone over time and with human passage during an epidemic. This analysis also reinforces large diversity of strains seen in cholera-endemic regions, and the apparent likelihood that infections in these areas can be caused by simultaneous infections with multiple strains (11).
Dr. Ali is a research associate professor at the Emerging Pathogens Institute and the Department of Environmental and Global Health, College of Public Health and Health Professions, University of Florida. His research interests are environmental persistence and evolution of V. cholerae and other Vibrio spp.
Acknowledgment
This study was supported by the University of Maryland Clinical Research Unit of the Food and Waterborne Diseases Integrated Research Network, which is supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (contract no. N01-AI-40014).
References
- Centers for Disease Control and Prevention. Update: cholera outbreak—Haiti, 2010. MMWR Morb Mortal Wkly Rep. 2010;59:1473–9.PubMedGoogle Scholar
- News BBC. Haiti cholera outbreak causes not clear, experts say. 2010 Oct 28 [cited 2010 Dec 3]. http://www.bbc.co.uk/news/world-latin-america-11618352
- Blake PA, Allegra DT, Snyder JD, Barrett TJ, McFarland M, Caraway CT, Cholera: a possible endemic focus in the United States. N Engl J Med. 1980;302:305–9. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Two cases of toxigenic Vibrio cholerae O1 infection after Hurricanes Katrina and Rita—Louisiana, October 2005. MMWR Morb Mortal Wkly Rep. 2006;55:31–2.
- Nandi B, Nandy RK, Mukhopadhyay S, Nair GB, Shimada T, Ghose AC. Rapid method for species-specific identification of Vibrio cholerae using primers targeted to the gene of outer membrane protein ompW. J Clin Microbiol. 2000;38:4145–51.PubMedGoogle Scholar
- Kumar P, Jain M, Goel AK, Bhadauria S, Sharma SK, Kamboj DV, A large cholera outbreak due to a new cholera toxin variant of the Vibrio cholerae O1 El Tor biotype in Orissa, eastern India. J Med Microbiol. 2009;58:234–8. DOIPubMedGoogle Scholar
- Nguyen BM, Lee JH, Cuong NT, Choi SY, Hien NT, Anh DD, Cholera outbreaks caused by an altered Vibrio cholerae O1 El Tor biotype strain producing classical cholera toxin B in Vietnam in 2007 to 2008. J Clin Microbiol. 2009;47:1568–71. DOIPubMedGoogle Scholar
- Alam M, Nusrin S, Islam A, Bhuiyan NA, Rahim N, Delgado G, Cholera between 1991 and 1997 in Mexico was associated with infection by classical, El Tor, and El Tor variants of Vibrio cholerae. J Clin Microbiol. 2010;48:3666–74. DOIPubMedGoogle Scholar
- Stine OC, Alam M, Tang L, Nair GB, Siddique AK, Faruque SM, Cholera epidemics in rural Bangladesh are the result of multiple small outbreaks. Emerg Infect Dis. 2008;14:831–3. DOIPubMedGoogle Scholar
- Ghosh R, Nair GB, Tang L, Morris JG Jr, Sharma NC, Ballal M, Epidemiological study of Vibrio cholerae using variable number of tandem repeats. FEMS Microbiol Lett. 2008;288:196–201. DOIPubMedGoogle Scholar
- Kendall EA, Chowdhury F, Begum Y, Khan AI, Li S, Thierer JH, Relatedness of Vibrio cholerae O1/O139 isolates from patients and their household contacts, determined by multilocus variable-number tandem repeat analysis. J Bacteriol. 2010;192:4367–76. DOIPubMedGoogle Scholar
- Choi SY, Lee JH, Jeon YS, Lee HR, Kim EJ, Ansaruzzaman M, Multilocus variable-number tandem repeat analysis of Vibrio cholerae O1 El Tor strains harbouring classical toxin B. J Med Microbiol. 2010;59:763–9. DOIPubMedGoogle Scholar
- Franco AA, Fix AD, Prada A, Paredes E, Palomino JC, Wright AC, Cholera in Lima, Peru, correlates with prior isolation of Vibrio cholerae from the environment. Am J Epidemiol. 1997;146:1067–75.PubMedGoogle Scholar
- Cameron DN, Khambaty FM, Wachsmuth IK, Tauxe RV, Barrett TJ. Molecular characterization of Vibrio cholerae O1 strains by pulsed-field gel electrophoresis. J Clin Microbiol. 1994;32:1685–90.PubMedGoogle Scholar
- Chin CS, Sorenson J, Harris JB, Robins WP, Charles RC, Jean-Charles RR, The origin of the Haitian cholera outbreak strain. N Engl J Med. 2011;364:33–42. DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleTable of Contents – Volume 17, Number 4—April 2011
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
J. Glenn Morris, Jr, Emerging Pathogens Institute, University of Florida, South Newell Dr, Bldg 62, PO Box 100009, Gainesville, FL 32610, USA
Top