Volume 17, Number 9—September 2011
Dispatch
Ciprofloxacin-Resistant Shigella sonnei among Men Who Have Sex with Men, Canada, 2010
Abstract
In 2010, we observed isolates with matching pulsed-field gel electrophoresis patterns from 13 cases of ciprofloxacin-resistant Shigella sonnei in Montréal. We report on the emergence of this resistance type and a study of resistance mechanisms. The investigation suggested local transmission among men who have sex with men associated with sex venues.
Shigella spp. are enteropathogen bacteria that are transmitted person-to-person and require a low infectious inoculum (1). Fluoroquinolones are among the first-choice antimicrobial drugs for treatment of Shigella spp. infections in adults (1), but resistance to these agents has been documented, primarily in Asia (2). Among men who have sex with men (MSM), Shigella spp. infection is, in most cases, sexually transmitted, and clusters are regularly reported (3–5). We investigated an outbreak of ciprofloxacin-resistant Shigella sonnei among MSM and studied its resistance mechanisms.
Laboratories report shigellosis to the Montreal public health department (Quebéc, Canada). When a cluster is suspected, isolates are sent to the provincial laboratory to conduct pulsed-field gel electrophoresis (PFGE) to identify links between patients.
In July 2010, microbiology services at the Hôpital Saint-Luc alerted the public health department to S. sonnei resistant to ciprofloxacin and trimethoprim/sulfamethoxazole and susceptible to ampicillin. The S. sonnei had been isolated 2 days apart from stool cultures of 2 HIV-positive MSM.
Public health officials sent a notice to physicians, clinics, and laboratories in Montréal to report the presence of ciprofloxacin-resistant S. sonnei among MSM and to describe the antimicrobial treatment with ampicillin or azithromycin, procedures for case reporting, and preventive measures (6). Confirmed cases were defined as infection by S. sonnei with resistance to ciprofloxacin and trimethoprim/sulfamethoxazole and susceptibility to ampicillin (later specified as pulsovar 72). Probable cases were defined as infection by S. sonnei with a resistance profile identical to that of confirmed cases but where PFGE was not conducted.
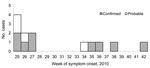
Retrospective searching of the notifiable disease database found ciprofloxacin-resistant S. sonnei with a different PFGE pattern that had been isolated in February 2010 from a female patient who had traveled to a country where shigellosis is highly prevalent. Hence, this case was not from this outbreak. The provincial laboratory searched their records to identify cases elsewhere in Québec. During June–October 2010, nine confirmed cases and 4 probable cases were identified in Montréal and the surrounding regions (Table 1). Most patients had an onset date from the end of June to mid-July 2010 (Figure 1). All 13 patients were interviewed. Most patients were men (11/13; 85%) with a mean age of 40 years (range 20–65 years). All male patients were MSM, and 4 (36%) of 11 reported being HIV positive. Travel to a European country during August 2010 was mentioned by 1 MSM patient. Eight (73%) of 11 MSM patients mentioned participation in anal sex or contact during the exposure period. The use of sex venues was indicated by 4/11 MSM patients, and 3 mentioned a common sex venue. In addition, 1 other MSM patient reported that his sex partners frequented the common sex venue. This suggests that unprotected anal sex, associated with local sex venues, was the primary mode of transmission.
Two female patients (45 and 50 years of age) were reported. S. sonnei was detected in a food sample from a restaurant where 1 female patient ate during the exposure period, but the isolate did not match the outbreak PFGE pattern and was not related to any known human patients. No epidemiologic links between the female and male patients could be identified.
The public health interventions included a weekly analysis of incident shigellosis infections, resistance profiles, and risk factors. Given the preponderance of infections among MSM visiting sex venues, kits of condoms, soap, and information on prevention were distributed at sex venues in August 2010 (3,6). Community-based organizations that work with MSM living with HIV/AIDS were contacted to disseminate information on preventive measures. As a potential effect, few cases were declared in September, although sporadic cases continued to appear until October 2010.
The resistance profile investigation identified 14 S. sonnei isolates from 13 patients by using commercial biochemical kit tests. Identification of S. sonnei from 9 patients was confirmed at the provincial laboratory. Antimicrobial susceptibility testing was done by agar dilution or disk diffusion method (7), Vitek 2 (bioMérieux, Marcy l’Étoile, France), or Etest (AB Biodisk, Solna, Sweden) (ampicillin, trimethoprim/sulfamethoxazole, and ciprofloxacin) for 14 isolates and by Etest (AB Biodisk) (azithromycin, cefotaxime, and tetracycline) and with nalidixic acid (30-μg disk) for 7 or 8 isolates. The susceptibility of S. sonnei isolates to antimicrobial agents is reported in Table 1 and Table 2.
PFGE was done by the provincial laboratory according to international standards set by the US Centers for Disease Control and Prevention (8). The XbaI and BlnI patterns were interpreted using the standards of Tenover et al. (9). The Salmonella enterica serotype Braenderup strain (H9812) was used as the size marker in each gel (10). Band position tolerances and optimization values of 1% were used for all analyses. Similarity coefficient was obtained within BioNumerics (www.applied-maths.com/bionumerics/bionumerics.htm) by calculating Dice coefficients. Cluster analysis was done by using with the unweighted pair group method with arithmetic averages. The S. sonnei isolates from 9 patients for whom typing was done were indistinguishable for the 2 enzymes (Figure 2). PulseNet Canada accession numbers for the isolate from our study are SSOXAI.0067 and SSOBNI.0040 for the XbaI and BlnI patterns, respectively.
For the study of the mechanisms of drug resistance, bacterial DNA was extracted using MasterPure Complete DNA Purification Kit (Epicenter Biotechnologies, Madison, WI, USA). The gyrA and parC genes were analyzed by direct DNA sequencing procedures as described (11) on an ABI Prism Genetic Analyzer 3130xl (Applied Biosystems, Foster City, CA, USA). The DNA sequences were converted into amino acid sequences by using the EMBOSS Transeq tool (European Molecular Biology–European Bioinformatics Institute), aligned by using ClustalW (DNAStar, Madison, WI, USA), and compared with that of the reference quinolone-susceptible strain (GenBank accession no. NC_008258). The 8 S. sonnei strains from 7 patients harbored the same nonsynonymous substitutions in comparison with the quinolone-susceptible reference strain: S83L and D87G for gyrA and S80I for parC. These amino acid substitutions have been previously associated with ciprofloxacin resistance in Escherichia coli (11) and in S. dysenteriae, S. flexneri, and S. boydii (2) but not in S. sonnei isolates.
Blood in stools or fever was reported by 9 (69%) of 13 patients (Table 1). Of the known treatment outcomes, 2 of the 4 patients treated with oral ampicillin had a negative stool culture 48 hours and 72 hours after completion. One of the 2 patients treated with oral amoxicillin experienced a clinical and microbiologic treatment failure 48 hours after completion, but a clinical and microbiologic cure was achieved after treatment with oral azithromycin. Two other patients were treated with azithromycin and 1 other with ciprofloxacin.
Sporadic ciprofloxacin-resistant Shigella has been infrequently documented (2,12–14). In India, ciprofloxacin-resistant S. dysenteriae, S. flexneri, and S. boydii have been isolated since 2002, and their fluoroquinolone-resistant mechanisms have been determined (2). Ciprofloxacin-resistant Shigella remains rare and was found among 0.2% of isolates in the United States (2000–2009) (12) and 0.5% of isolates in Canada (1997–2000) (13). In the United States, 10 ciprofloxacin-resistant Shigella spp. isolates (6 S. flexneri, 3 S. sonnei, and 1 Shigella spp.) were documented by the National Antimicrobial Resistance Monitoring System (2000–2009) (12). In New York, NY, in 2006, ciprofloxacin resistance was detected among 4 S. sonnei and 1 S. flexneri acquired locally (14). In 2010 in South Carolina, ciprofloxacin-resistant Shigella flexneri 2a was isolated from 3 patients (15).
We report the suspected transmission of ciprofloxacin-resistant S. sonnei, among MSM in Montreal, Québec. Some authors suggest the antimicrobial drug treatment of all patients infected with Shigella spp (1), but others disagree with this recommendation (14). It is essential that physicians request bacterial stool cultures when Shigella spp. enteric infection is suspected in MSM even without blood in stools or fever.
Dr Gaudreau is a clinical microbiologist and infectious diseases physician at Centre Hospitalier de l’Université de Montréal-Hôpital Saint-Luc in Montreal and a clinical titular professor at the Département de Microbiologie et Immunologie de l’Université de Montréal. Her main research interests are antimicrobial drug susceptibility and epidemiology of enteric bacteria.
Acknowledgment
We thank Michèle Tremblay, Elisabeth Lacombe, Patricia Hudson, Sylvie Habash, Suzanne Rajotte, Michel Poisson, Claude Lemieux, M.-D. Baptiste-Desruisseaux, Johanne Ismaïl, the medical microbiologists from Clinique l’Actuel and the hospitals Verdun and Maisonneuve-Rosemont, Pierre-Boucher, Jean-Talon for their assistance.
References
- DuPont HL. Shigella species (Bacillary dysentery). In: Mandell GL, Bennett JE, Dolin R, editors. Principles and practice of infectious diseases, 7th ed. Philadelphia: Elsevier Churchill Livingston; 2010. p. 2905–10.
- Pazhani GP, Niyogi SK, Singh AK, Sen B, Taneja N, Kundu M, Molecular characterization of multidrug-resistant Shigella species isolated from epidemic and endemic cases of shigellosis in India. J Med Microbiol. 2008;57:856–63. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Shigella sonnei outbreak among men who have sex with men—San Francisco, California, 2000–2001. MMWR Morb Mortal Wkly Rep. 2001;50:922–6.PubMedGoogle Scholar
- Morgan O, Crook P, Cheasty T, Jiggle B, Giradon I, Hughes H, Shigella sonnei outbreak among homosexual men, London. Emerg Infect Dis. 2006;12:1458–60.PubMedGoogle Scholar
- Gaudreau C, Bruneau A, Ismaïl J. Outbreak of Shigella flexneri and Shigella sonnei enterocolitis in men who have sex with men, Québec, 1999 to 2001. Can Commun Dis Rep. 2005;31:85–90.PubMedGoogle Scholar
- Direction de santé publique, Agence de la santé et des services sociaux de Montréal. Sexual transmission of shigellosis[update 2010 Aug 9] [cited 2011 Mar 9]. http://www.santepub-mtl.qc.ca/its/shigellose/indexenglish.html
- Clinical and Laboratory Standards Institute. Antimicrobial susceptibility testing: twentieth informational supplement; no. M100–S20 Vol. 30 No. 1. Wayne (PA): The Institute; 2010.
- Ribot EM, Fair MA, Gautom R, Cameron DN, Hunter SB, Swaminathan B, Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157:H7, Salmonella and Shigella for PulseNet. Foodborne Pathog Dis. 2006;3:59–67. DOIPubMedGoogle Scholar
- Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–9.PubMedGoogle Scholar
- Hunter SB, Vauterin P, Lambert-Fair MA, Van Duyne MS, Kubota K, Graves L, Establishment of a universal size standard strain for use with the PulseNet standardized pulsed-field gel electrophoresis protocols: converting the national databases to the new size standard. J Clin Microbiol. 2005;43:1045–50. DOIPubMedGoogle Scholar
- Chu YW, Cheung TK, Wong CH, Tsang GK, Lee K, Lau SS, Quinolone resistance and correlation to other antimicrobial resistances in faecal isolates of Escherichia coli in Hong Kong. Chemotherapy. 2008;54:274–8. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. 2010. National Antimicrobial Resistance Monitoring System—enteric bacteria (NARMS) 2009 annual report [cited 2011 Jun 29]. http://www.cdc.gov/narms/pdf/NARMSAnnualReport2009_508.pdf
- Martin LJ, Flint J, Ravel A, Dutil L, Doré K, Louie M, Antimicrobial resistance among Salmonella and Shigella isolates in five Canadian provinces (1997 to 2000). Can J Infect Dis Med Microbiol. 2006;17:243–50.PubMedGoogle Scholar
- Wong MR, Reddy V, Hanson H, Johnson KM, Tsoi B, Cokes C, Antimicrobial resistance trends of Shigella serotypes in New York City, 2006–2009. Microb Drug Resist. 2010;16:155–61. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Emergence of Shigella flexneri 2a resistant to ceftriaxone and ciprofloxacin—South Carolina, October 2010. MMWR Morb Mortal Wkly Rep. 2010;59:1619.PubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 17, Number 9—September 2011
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Christiane Gaudreau, Microbiologie Médicale et Infectiologie, CHUM-Hôpital Saint-Luc, 1058 rue Saint-Denis, Montreal, Québec H2X 3J4, Canada
Top