Volume 18, Number 5—May 2012
Dispatch
Unsuspected Rickettsioses among Patients with Acute Febrile Illness, Sri Lanka, 2007
Abstract
We studied rickettsioses in southern Sri Lanka. Of 883 febrile patients with paired serum samples, 156 (17.7%) had acute rickettsioses; rickettsioses were unsuspected at presentation. Additionally, 342 (38.7%) had exposure to spotted fever and/or typhus group rickettsioses and 121 (13.7%) scrub typhus. Increased awareness of rickettsioses and better tests are needed.
Globally, rickettsioses are increasingly recognized as causes of undifferentiated fever. Paired serum samples are infrequently obtained, but testing acute-phase serum alone is insensitive (IgG is initially absent) and nonspecific (IgG can persist for years, and IgM results represent cross-reactions).
Sentinel studies in Malaysia (1), Thailand (2), India (3), Laos (4), and Nepal (5) suggest that scrub and murine typhus are frequent and that misdiagnosis as enteric fever results in ineffective therapy (5). Unrecognized rickettsial species are likely present in Sri Lanka, an island connected to the southern tip of India by an underwater 30-km land bridge. Kularatne reported acute rickettsioses diagnosed by using only acute-phase serum IgM in 56 of 118 patients who had fever in hilly central Sri Lanka (6); another study in the Western Province confirmed few (5/31cases) of suspected rickettsioses (7). Both studies were limited by selective enrollment. To characterize rickettsioses among undifferentiated febrile illnesses in southern Sri Lanka, we prospectively studied patients who came to a large hospital.
Consecutive patients >2 years of age with fever (>38°C tympanic) who came to Teaching Hospital Karapitiya were enrolled (8). Standardized epidemiologic and clinical data and blood were obtained during acute illness and 2–4 weeks later. During the study (March–October 2007), the atmospheric temperature ranged from 27.5°C–32°C (high) to 24°C–26°C (low), and rainfall was variable (mean 301 mm/mo, range 36–657 mm/mo).
Because rickettsial species broadly cross-react within groups (9,10), paired serum samples were tested by using an IgG indirect immunofluorescence assay (IFA) and Rickettsia rickettsii and R. typhi antigens (Focus Diagnostics, Cypress, CA, USA) to identify infections with spotted fever group (SFGR) and typhus group (TGR) rickettsial infections. Serum samples reactive at a titer of 80 were considered potentially positive and were titered.
To identify scrub typhus (ST) infections, we tested paired serum samples using IgG ELISA as described (11), except for use of recombinant antigens (0.2 µg each of r56 Chimeric1, Gilliam, and Kato strains) to detect antibodies to Orientia tsutsugamushi. Comparative blind testing of 200 serum samples with an established (pooled-antigen) quantitative assay enabled validation (12).
Acute rickettsioses (SFGR, TGR, and ST) required a >4-fold rise in specific IgG titer or its equivalent; patients with equal SFGR and TGR convalescent-phase titers were SFGR/TGR group-indeterminate. IgG (titer >160) in acute-phase serum defined rickettsial exposure (seroprevalence). Stata IC version 11.0 (StataCorp LP, College Station, TX, USA) was used for analyses.
We analyzed paired serum samples for rickettsioses for 883 (81.9%) of 1,079 patients. Median acute–convalescent phase follow-up was 21 days (intraquartile range 15−33 days). Patients with and without paired serum samples were comparable (8). Acute rickettsioses were documented in 156 (17.7%) patients (Table 1). The increase in convalescent-phase geometric mean titer was 14-fold (845) for SFGR, 17-fold (920) for TGR, and 11-fold (951) for SFGR/TGR rickettsiae.
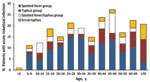
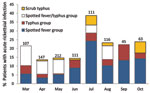
Acute rickettsioses were found in 19.7% of patients >18 years of age and 10.5% of patients <18 years of age (p = 0.003); patients with rickettisoses were older than those without rickettsioses (median age 34.5 vs. 28.0 years; p = 0.005) (Figure 1). Among patients <18 years of age, acute rickettsioses were more common in male than in female patients (14.6% vs. 5.8%; p<0.05). Patients with acute ST alone were older than those with other rickettsioses and those without rickettsioses (median 36.5 vs. 34.4 vs. 28.0 years; p = 0.02) and more likely to report rice paddy exposure (44.4% vs. 15.1% vs. 9.3%; p = 0.001). More acute rickettsioses occurred during the July–October rains (70.6% of ST infections and 59.7% of other rickettsioses), whereas more nonrickettsial infections (67.0%) occurred during the drier period of March–June (p<0.0001) (Figure 2).
Acute rickettsioses were clinically similar to each other and to nonrickettsial febrile illnesses, except for frequency of cough, oliguria, and conjunctival suffusion (Table 1). Except for a higher temperature with SFGR than with TGR infection (mean 38.6 vs. 38.2°C; p = 0.006), no feature differentiated these rickettsial infections. Conjunctival suffusion was more common (p = 0.004) with ST (35.3%) than with SFGR/TGR (12.8%) or no rickettsiosis (12.8%).
Antecedent antimicrobial drug use was commonly reported in patients with (45/115 [39.1%]) and without (195/536 [36.4%]; p = 0.58) rickettsioses. Amoxicillin and cephalosporins were administered most frequently in both groups (16.8% and 20.3%; p = 0.40), but infrequent administration of doxycycline (0.9% and 1.1%; p = 0.82) was recorded for 813 patients with paired serum samples, including 139 with acute rickettsioses. Rickettsioses were rarely clinically identified when present (3/139, sensitivity 2.2%, 95% CI 0.447%– 6.2%). Rickettsioses were also infrequently confirmed when diagnosed clinically (3 SFGR rickettsioses among 9 with suspected scrub typhus; positive predictive value 33.3%, 95% CI 7.5– 70.1) and rarely treated appropriately (2/9 given doxycycline, 1 with SFGR rickettsiosis). Patients with rickettsioses were hospitalized longer than others (median 5 vs. 4 days; p = 0.01), although the proportion hospitalized was similar. No one with confirmed rickettsioses died, but 11 of 12 patients died before follow-up.
At enrollment, 292 (33.1%) patients had IgG-confirmed rickettsial exposure. However, only 59 (20.2%) had a 4-fold increase in titer, and 97 (62.2%) of 156 acute infections were identified as rickettsioses by seroconversion. Exposures to rodents and rice paddies were associated with TGR rickettsiae and O. tsutsugamushi IgG (Table 2). Farmers were more likely (p = 0.01) to have IgG against O. tsutsugamushi (5.7%) than SFGR/TGR rickettsiae IgG alone (4.3%) or no rickettsial IgG (1.5%). If IgG titer >160 at either sampling were used to denote rickettsial exposure (preexisting or acute), 342 (38.7%) patients had exposure to SFGR and/or TGR rickettsiae. If a lower titer (>80) were used, 398 (45.1%) had such exposure. Similarly, 121 (13.7%) patients had either acute- or convalescent-phase IgG against O. tsutsugamushi.
We documented endemic rickettsioses as a major cause of acute febrile illness in southern Sri Lanka. Epidemiologic features could not differentiate acute infection from prior exposure. Rickettsioses were infrequently suspected and not treated empirically when suspected. Underrecognition of rickettsioses could reflect nonspecific clinical features, limited diagnostic tools, lack of awareness that rickettsioses occur or cause severe illness, and absence of evidence-based local algorithms for acute febrile illness.
Studies in neighboring regions have used flawed methods, including spectrum bias, small sample size, selective enrollment, and testing acute-phase serum only. In many instances, clinical features were not predictive (5,13); however, older age was reported with rickettsioses (14) and lymphadenopathy and rapid respiratory rate with ST compared with TGR rickettsiosis (4). Although part of the rickettsial triad, rash is often absent initially (10) and among unselected febrile patients (5,14). SFGR/TGR cross-reactions and apparent co-infections could also impair clinical differentiation of specific rickettsioses. Laboratory abnormalities associated with ST (3) could reflect disease severity, not etiology, and such testing is often unavailable. Divergent conclusions might reflect different study populations, diagnostic criteria, reference groups, features evaluated, or real geographic differences.
Our estimate of rickettsioses is conservative. Confirmation required follow-up (return or home visit); the US Centers for Disease Control and Prevention no longer accepts a single high titer to confirm R. rickettsii infection (10). We required a 4-fold rise in titer even for seroconversions because IFA results are subjective, even among experts. We used R. rickettsii as a surrogate antigen, which could be less sensitive for detecting local SFGR. We chose ELISA for ST because a commercial IFA was not available and IFA as a standard for ST has been questioned (15).
Optimal clinical management of acute rickettsioses requires development of locally tested, evidence-based algorithms for acute febrile illness. Better diagnostic tests are needed to identify new species, elucidate vector–host relationships, and enable appropriate therapy. Molecular approaches hold promise but will require prospective validation.
Dr Reller is a pediatric and adult infectious diseases physician, medical microbiologist, and clinical investigator at Johns Hopkins University School of Medicine. Her main research interests include study of the epidemiology of acute febrile illness and its improved diagnosis.
Acknowledgments
We thank the members of the microbiology laboratory at the Medical Faculty, University of Ruhuna, and the clinical staff at Teaching Hospital Karapitya for their assistance; P. L. Ariyananda for support of the study; Cynthia Binanay for project management; our clinical research team, especially Vathsala Abeygunawardane, for enrollment of patients; and Wei Mei Ching and Allen Richards for providing antigens and protocols for assessment of scrub typhus.
Patient enrollment was supported by the Hubert-Yeargan Center for Global Health and the Duke University Medical Center Chancellor’s Tsunami Relief Fund. Laboratory testing and M.E.R. were supported by a Johns Hopkins Center for Global Health Junior Faculty grant; a Clinician Scientist Career Development Award from Johns Hopkins School of Medicine; and the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K23AIO83931).
References
- Brown GW, Shirai A, Jegathesan M, Burke DS, Twartz JC, Saunders JP, Febrile illness in Malaysia—an analysis of 1,629 hospitalized patients. Am J Trop Med Hyg. 1984;33:311–5.PubMedGoogle Scholar
- Suttinont C, Losuwanaluk K, Niwatayakul K, Hoontrakul S, Intaranongpai W, Silpasakorn S, Causes of acute, undifferentiated, febrile illness in rural Thailand: results of a prospective observational study. Ann Trop Med Parasitol. 2006;100:363–70. DOIPubMedGoogle Scholar
- Varghese GM, Abraham OC, Mathai D, Thomas K, Aaron R, Kavitha ML, Scrub typhus among hospitalised patients with febrile illness in south India: magnitude and clinical predictors. J Infect. 2006;52:56–60. DOIPubMedGoogle Scholar
- Phongmany S, Rolain JM, Phetsouvanh R, Blacksell SD, Soukkhaseum V, Rasachack B, Rickettsial infections and fever, Vientiane, Laos. Emerg Infect Dis. 2006;12:256–62. DOIPubMedGoogle Scholar
- Murdoch DR, Woods CW, Zimmerman MD, Dull PM, Belbase RH, Keenan AJ, The etiology of febrile illness in adults presenting to Patan hospital in Kathmandu, Nepal. Am J Trop Med Hyg. 2004;70:670–5.PubMedGoogle Scholar
- Kularatne SA, Edirisingha JS, Gawarammana IB, Urakami H, Chenchittikul M, Kaiho I. Emerging rickettsial infections in Sri Lanka: the pattern in the hilly Central Province. Trop Med Int Health. 2003;8:803–11. DOIPubMedGoogle Scholar
- Premaratna R, Loftis AD, Chandrasena TG, Dasch GA, de Silva HJ. Rickettsial infections and their clinical presentations in the Western Province of Sri Lanka: a hospital-based study. Int J Infect Dis. 2008;12:198–202. DOIPubMedGoogle Scholar
- Reller ME, Bodinayake C, Nagahawatte A, Devasiri V, Kodikara-Arachichi W, Strouse JJ, Leptospirosis as frequent cause of acute febrile illness in southern Sri Lanka. Emerg Infect Dis. 2011;17:1678–84. DOIPubMedGoogle Scholar
- Hechemy KE, Raoult D, Fox J, Han Y, Elliott LB, Rawlings J. Cross-reaction of immune sera from patients with rickettsial diseases. J Med Microbiol. 1989;29:199–202. DOIPubMedGoogle Scholar
- Chapman AS, Bakken JS, Folk SM, Paddock CD, Bloch KC, Krusell A, Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, ehrlichioses, and anaplasmosis—United States: a practical guide for physicians and other health-care and public health professionals. MMWR Recomm Rep. 2006;55:1–27.PubMedGoogle Scholar
- Chen HW, Zhang Z, Huber E, Mutumanje E, Chao CC, Ching WM. Kinetics and magnitude of antibody responses against the conserved 47-kilodalton antigen and the variable 56-kilodalton antigen in scrub typhus patients. Clin Vaccine Immunol. 2011;18:1021–7. DOIPubMedGoogle Scholar
- Coleman RE, Sangkasuwan V, Suwanabun N, Eamsila C, Mungviriya S, Devine P, Comparative evaluation of selected diagnostic assays for the detection of IgG and IgM antibody to Orientia tsutsugamushi in Thailand. Am J Trop Med Hyg. 2002;67:497–503.PubMedGoogle Scholar
- Duffy PE, Le Guillouzic H, Gass RF, Innis BL. Murine typhus identified as a major cause of febrile illness in a camp for displaced Khmers in Thailand. Am J Trop Med Hyg. 1990;43:520–6.PubMedGoogle Scholar
- Ellis RD, Fukuda MM, McDaniel P, Welch K, Nisalak A, Murray CK, Causes of fever in adults on the Thai–Myanmar border. Am J Trop Med Hyg. 2006;74:108–13.PubMedGoogle Scholar
- Blacksell SD, Bryant NJ, Paris DH, Doust JA, Sakoda Y, Day NP. Scrub typhus serologic testing with the indirect immunofluorescence method as a diagnostic gold standard: a lack of consensus leads to a lot of confusion. Clin Infect Dis. 2007;44:391–401. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 18, Number 5—May 2012
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Megan E. Reller, Johns Hopkins Medical Institutions, 720 Rutland Ave, Ross 624, Baltimore, MD 21205, USA
Top