Volume 18, Number 5—May 2012
Letter
Novel Prion Protein in BSE-affected Cattle, Switzerland
Cite This Article
Citation for Media
To the Editor: In a recent issue of Emerging Infectious Diseases, Seuberlich et al. (1) reported a novel prion protein in cattle with bovine spongiform encephalopathy (BSE). Two cows in Switzerland, 8 and 15 years of age, tested positive in 2 approved screening tests, the PrioSTRIP test and the Prionics Check WESTERN (Prionics, Zurich, Switzerland). According to World Organisation for Animal Health guidelines, the 2 cattle are considered BSE positive. Histopathologic and immunohistochemical results were inconclusive because the tissues were severely autolyzed. Clinical signs were absent or the clinical history was not known.
After further analysis of brain tissues by using several monoclonal antibodies in a Western blot (WB), the authors concluded that they had identified an N-terminal truncated protease-resistant prion protein (PrPres) fragment that differs from the PrPres fragments in 3 known types of BSE. No reference was made to the existence of N-truncated fragments, such as C1, of the normal prion protein PrPC, which have been reported for humans (2,3), mice (4), and cattle and other ruminants (5). The pattern in the WB of the novel prion protein (1) appears similar to that of the fragment C1 of the normal prion protein (2–5). The C1 fragment is more protease resistant than the intact PrPC fragment because the protein part is more protected by the polysaccharide residues. Could it be that in the case of the severely autolyzed tissues of the cows in Switzerland, the proteinase K might already have been weakened or inhibited and when combined with the higher protease resistance of the C1 fragment, the digestion was incomplete?
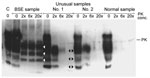
Ten years ago, I looked at nonspecific, unusual samples from fallen stock cattle in New Zealand. Samples from these cattle had been confirmed as negative by paraffin-embedded tissue blot (University of Göttingen, Göttingen, Germany), sodium phosphotungstic acid precipitation, followed by WB (European Union Reference Laboratory for Transmissible Spongiform Encephalopathies, Veterinary Laboratories Agency, New Haw, UK), Prionics WB (Prionics AG, Zurich, Switzerland), histopathologic examination, and immunohistochemical testing (Veterinary Laboratories Agency). We became aware of such samples when the proteinase K digestion did not work properly (Figure). Unusual samples 1 and 2 contained increased amounts of a truncated fragment of normal PrPC, which was digested completely after the proteinase K concentration was increased.
I am convinced that the novel PrPres described in article by Seuberlich et al. (1) is indeed a truncated fragment of the normal bovine PrPC protein. Therefore, I would like to ask the editor to address the following issues with the authors: Why were no references to truncated fragments of PrPC made in their article? Why was no WB analysis performed in which the novel PrPres was shown next to normal, undigested PrPC for band-size comparison? Why were no WB analyses shown in which the proteinase K concentration was increased?
It is laudable that in vivo transmission studies using transgenic mouse models and cattle are under way, which will sort out these findings conclusively. I expect that no disease development will be shown. Meanwhile, announcing new types of BSE is purely speculation.
References
- Seuberlich T, Gsponer M, Drögemüller C, Polak MP, McCutcheon S, Heim D, Novel prion protein in BSE-affected cattle, Switzerland. Emerg Infect Dis. 2012;18:158–9. DOIPubMedGoogle Scholar
- Chen SG, Teplow DB, Parchi P, Teller JK, Gambetti P, Autilio-Gambetti L. Truncated forms of the human prion protein in normal brain and in prion diseases. J Biol Chem. 1995;270:19173–80. DOIPubMedGoogle Scholar
- Pan T, Li R, Wong B-S, Liu T, Gambetti P, Sy M-S. Heterogeneity of normal prion protein in two-dimensional immunoblot: presence of various glycosylated and truncated forms. J Neurochem. 2002;81:1092–101. DOIPubMedGoogle Scholar
- Mangé A, Béranger F, Peoc’h K, Onoder T, Frobert Y, Lehmann S. Alpha- and beta- cleavages of the amino-terminus of the cellular prion protein. Biol Cell. 2004;96:125–32. DOIPubMedGoogle Scholar
- Klingeborn M. The prion protein in normal cells and disease. Studies on the cellular processing of bovine PrPC and molecular characterization of the Nor98 prion [dissertation]. Uppsala (Sweden): Swedish University of Agricultural Sciences; 2006.
Figure
Cite This ArticleRelated Links
In Response: Dr Kittelberger comments on our recent report of 2 cows in Switzerland that were classified as positive for bovine spongiform encephalopathy (BSE), according to the established criteria (1,2). He raises concerns that the unusual prion protein signature in Western blot (WB) in these cows represents a physiologic prion protein (PrPC) fragment, inefficiently degraded by proteinase K (PK), termed C1. Certainly the effects of tissue autolysis on PK activity and the molecular prion protein signature are of particular concern and deserve full consideration in data interpretation. In our study, molecular mass comparisons between PrPC in non-PK–treated brain tissue of healthy cattle and the prion protein in samples from the 2 aberrant cows with BSE in WB were considerably hindered by overlapping C1- and full-length PrPC bands in the non-PK–treated samples and did not allow for a robust conclusion (T. Seuberlich, unpub. data). It is noteworthy that the Prionics Check WESTERN (Prionics, Zurich, Switzerland) test has been extensively validated in terms of the diagnostic specificity, also on severely autolytic specimens (3–5). In none of these studies was a similar prion protein signature observed. We therefore considered it unlikely that the findings in the cases from Switzerland resulted from tissue autolysis.
Dr Kittelberger provides data from New Zealand cattle that revealed a similar prion protein signature in WB. He assumes that these animals had a negative BSE status and that the PK digestion in the WB did not work properly, which is supported by results from other diagnostic techniques. However, information about the degree of autolysis of these samples is missing, and, most notably, whether these findings are correlated with prion infectivity is not known. Strikingly, in contrast to the results for the samples from Switzerland, the samples from New Zealand are reported to be negative in the Prionics Check WESTERN. It would be fascinating to perform a side-by-side analysis of the samples from Switzerland and from New Zealand to determine whether the banding characteristics in both groups are identical. Studies are under way in our laboratory to further investigate the effect of tissue autolysis on PK activity and PrPC degradation under experimental conditions. If our findings turn out to be the result of inhibited PK activity in BSE-negative cattle samples, the current diagnostic criteria might require revision. As long as the results of these experiments and the ongoing transmission studies are not available, we can neither confirm nor reject a novel type of BSE.
References
- Seuberlich T, Gsponer M, Drögemüller C, Polak MP, McCutcheon S, Heim D, Novel prion protein in BSE-affected cattle, Switzerland. Emerg Infect Dis. 2012;18:158–9. DOIPubMedGoogle Scholar
- Kittelberger R. Novel prion protein in BSE-affected cattle, Switzerland [letter]. Emerg Infect Dis. 2012;18:890–2 .DOIGoogle Scholar
- Schaller O, Fatzer R, Stack M, Clark J, Cooley W, Biffiger K, Validation of a western immunoblotting procedure for bovine PrP(Sc) detection and its use as a rapid surveillance method for the diagnosis of bovine spongiform encephalopathy (BSE). Acta Neuropathol. 1999;98:437–43. DOIPubMedGoogle Scholar
- European Food Safety Authority. Scientific report on the evaluation of seven new rapid post mortem BSE tests. EFSA Scientific Report. 2004;18:1–13 [cited 2012 Mar 1]. http://www.efsa.europa.eu/en/scdocs/doc/18r.pdf
- Office International des Epizooties. OIE procedure for validation and certification of diagnostic assays. Abstract sheet for the Prionics-AG Check WESTERN [cited 2012 Mar 1]. http://www.oie.int/fileadmin/Home/eng/Our_scientific_expertise/docs/pdf/Abstract_20sheet_OIE_20Register_PrionicsWB_v1.pdf
Table of Contents – Volume 18, Number 5—May 2012
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors:
Top