Volume 18, Number 7—July 2012
Letter
Treatment Duration for Patients with Drug-Resistant Tuberculosis, United States
Figure
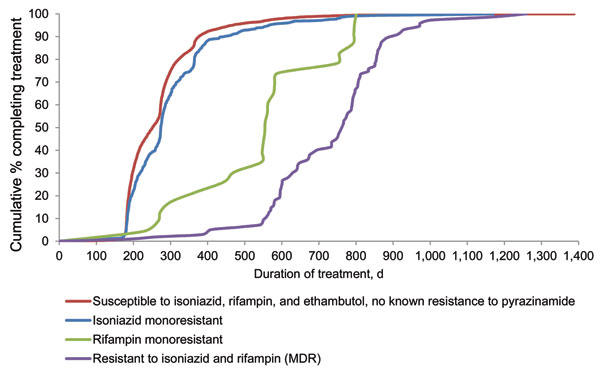
Figure. . . Treatment duration, by drug-resistance pattern, among reported tuberculosis case-patients who completed treatment, United States, 2006. Cases were among patients who were alive and initiated therapy at diagnosis and who had start and end therapy dates as well as results for initial drug susceptibility testing to isoniazid, rifampin, and ethambutol. Susceptibility testing was conducted on culture-positive Mycobacterium tuberculosis isolates from any specimen type.
Page created: June 08, 2012
Page updated: June 08, 2012
Page reviewed: June 08, 2012
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.